CO2 Solubility in Shanxi Formation Water of Ordos Basin
-
摘要: 实施CO2的地质储存是目前公认的减缓全球变暖的有效途径之一.潜在的储存场所包括衰竭的油气藏、深部不可开采煤层及深部咸水层.其中, 深部咸水层储存潜力最大.在发挥作用的诸多机理中, 溶解埋存具有埋存量大、作用时间较长以及安全性高的特点.在评价深部咸水含水层CO2溶解储存潜力时, 溶解度是一个关键参数.提出了测定咸水含水层地层水CO2溶解度的方法, 并将其实际应用于鄂尔多斯盆地山西组地层水.鄂尔多斯盆地是我国重要的能源基地, CO2排放量大, 排放浓度高.采集了野外实地水样, 进行了化学成分分析, 并人工合成该水样; 测定了40~80 ℃、8~12 MPa条件下CO2在该水样中的溶解度, 其结果可为评价鄂尔多斯盆地深部咸水含水层埋存能力提供依据.Abstract: Geological storage is one of the most effective means to reduce the anthropogenic greenhouse gas emissions to mitigate the worsening global warming. Depleted oil-gas reservoirs, coal seams and deep saline aquifers are potential sites for CO2 geological storage of which saline aquifer has the greatest potential for sequestration. Among the many effective mechanisms, dissolving storage is characterized by large storage capacity, long action time and high safety. When evaluating the storage capacity of a deep saline aquifer, CO2 solubility becomes a key parameter. In this paper, an experimental method is proposed and used to measure the CO2 solubility in Shanxi Formation water. Ordos Basin is an important energy base for China which releases a lot of high concentration CO2. Studies show CO2 geological storage is possible in Ordos Basin since its Shanxi Formation forms many source-reservoir-cap assemblages, and it is of great importance both in theory and practice to probe into CO2 solubility in Shanxi Formation water of Ordos Basin. In this paper, chemical composition of Shanxi Formation water collected from the Ordos Basin were analyzed. CO2 solubilities in the artificial synthetic Shanxi Formation waterwere measured at 40-80℃, 8-12 MPa pressure. The results can be used for the evaluation of the CO2 storage capacity in deep saline aquifer of Ordos Basin.
-
Key words:
- geological storage /
- Ordos basin /
- solubility /
- groundwater /
- hydrogeology
-
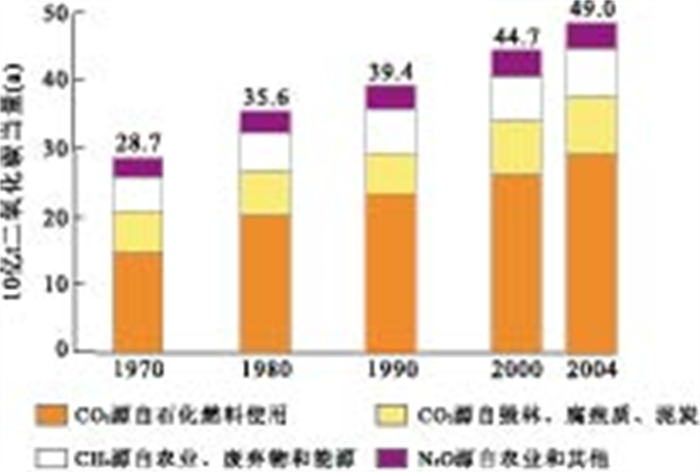
自工业革命以来,由于人类对化石燃料的过度依赖,导致工业生产和人类生活过程中产生的温室气体日益增多,温室效应正在严重威胁着人类的生存环境.据IPCC(政府间气候变化专门委员会)2007年第四次报告显示,在众多温室气体中,CO2对温室效应的贡献最大.1970年至2004年间,全球CO2的排放量从210亿t增加到380亿t,增加了约80%(图 1).全球变暖已经引起了一系列的环境地质问题,如气温升高、冰川融化、海平面上涨、极端天气现象以及由此导致的生态系统破坏、粮食减产、病虫害肆虐等.
实施CO2地质储存是目前大多数科学家公认的减少CO2排放量的有效方法之一.根据国际能源署(IEA)以及政府间气候变化专业委员会(IPCC)的评估,在可能实施二氧化碳地质储存的场所中,深部咸水含水层储存潜力最大.沈平平和廖新维(2009)阐述了CO2的3种主要捕集方式:(1)自由CO2,主要是超临界状态(物理捕集);(2)溶解在地下水中(水力捕集);(3)与岩石反应生成碳酸盐沉淀以矿物形式埋存(矿物捕集).在储存的前期阶段,物理捕集是主要的捕集机制.随着时间的推移,水力捕集和矿物捕集成为CO2长期储存的主要方式.李小春等(2006)利用溶解度法计算的中国咸水含水层CO2储存容量约为1.43505×1011t,相当于中国大陆地区2003年CO2排放总量的40.5倍,其中鄂尔多斯盆地的储量约为73.17×108t.
鄂尔多斯盆地是一个多种资源能源富集的大型沉积盆地,也是我国区域性的大型能源化工规划基地,目前已设立了5个能源基地规划区,已建和在建的有多个大型煤化工项目(任相坤等,2010).据预计所有在建项目投产后,每年将排放大约4000×104t浓度高于80%的CO2.已有研究表明,鄂尔多斯盆地属于整体沉降,盆地内部地质构造不发育,地震活动少且拥有多套储盖层组合,初步判断具有封存CO2的潜力.其中二叠系山西组位于地下2232~2316m,岩性为中-细砂岩、泥岩互层夹数层煤层,具有良好的储盖层组合,满足CO2在深部咸水含水层的储存要求.因此对鄂尔多斯盆地山西组的CO2地质储存能力的研究具有重要的实际意义.
Li et al.(2005)提出了CO2溶解储存容量的计算公式:
SCO2=aAhηnRρwMCO2, (1) 其中:a为可用于储存CO2的咸水含水层平面分布范围占总盆地的比例;A为分区面积(m2);h为沉积层厚度(m);η为含水层厚度占总沉积层的比例;n为孔隙度;R为地层水中CO2溶解度(mol/kg);ρw为储存深度条件下饱和CO2的咸水密度(kg/m3);M为CO2的摩尔质量.
由公式(1)可知,CO2的溶解度是计算含水层CO2溶解储存量的关键参数.其值是由压力、温度以及地层水共同决定的.目前国内外很多学者通过不同的方法研究不同温度压力条件下CO2的溶解度.
Wiebe and Gaddy(1939, 1940)研究了12~40℃,以及50、75、100℃,压力最高700atm(1atm=101325Pa)时CO2在纯水中的溶解度;Ellis and Golding(1963)通过实验测得了175~335℃时CO2在纯水以及NaCl溶液(浓度分别为0.5,1,2mol/L)中的溶解度;Liu et al.(2011)的研究给出了CO2在NaCl、KCl、CaCl2及其混合物中的溶解度;Teng and Yamasaki(1998)以合成海水为研究对象,研究了在0~20℃、6.44~29.49MPa条件下CO2在合成海水中的溶解度;Portier and Rochelle(2005)给出了不同温度压力内(0~300℃,1×105~300×105Pa)CO2在纯水、NaCl以及人工合成Utsira地层水中的溶解度值,并实际运用到Sleipher项目中;Duan and Sun(2003)以液相粒子相互作用理论以及气相状态方程为基础,建立了一个热力学模型,很好地模拟了0~260℃,0~200MPa条件下CO2在0~4m深度NaCl溶液中的溶解度;Darwish and Hilal(2010)则以Setschenov模型为基础,提出了一个新的模型,用来模拟27~227℃、5~200MPa条件下CO2在H2O-NaCl体系中的溶解度.
目前国内外对CO2溶解度的研究大多数都以纯水及各种盐溶液为研究对象,针对具体地层水中CO2溶解度的研究较少,尤其缺乏针对我国各地层水的CO2溶解能力研究.本文以鄂尔多斯盆地山西组地层水为研究对象,在对该地下水进行化学成分分析后,人工合成该水样,测试不同温度压力条件下CO2在其中的溶解度.
1. 实验材料和方法
1.1 实验材料
实验所用CO2纯度为99.95%,由北京如原如泉公司提供.NaCl、KCl、MgCl2、CaCl2、NaHCO3、NaOH、HCl均为北京试剂厂分析纯试剂.
1.2 实验条件
根据曾荣树等(2004)提出适宜CO2地质储存的地热梯度(25~35℃/km)、压力梯度(10.5MPa/km)、埋存深度(>800m)以及侯光才和张茂省等(2008)对鄂尔多斯盆地地热梯度研究结果(25~30℃/km),本文将研究范围确定为40~80℃、8~12MPa.
1.3 实验装置及过程
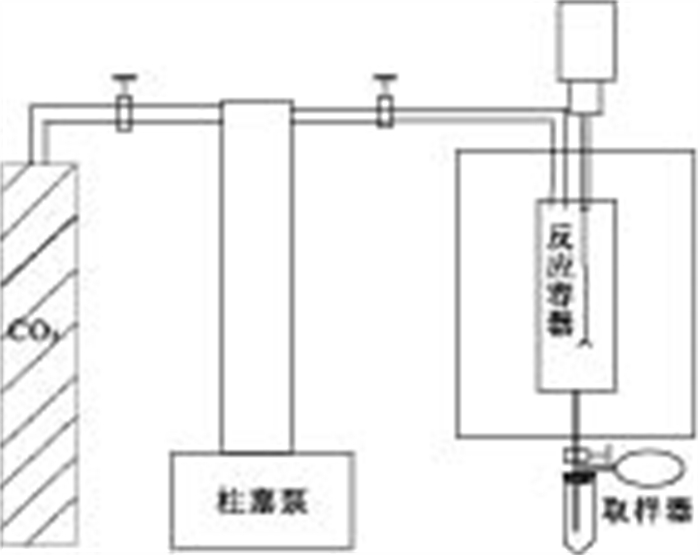
实验采用如图 2所示的装置.装置主体部分为高温高压反应釜,釜内为容积600mL的钛合金容器,是本次实验的主要反应场所,实验中人工合成山西组地下水就放置在该容器中,容器接有温度和压力传感器,可随时观察或控制釜内的温度及压力;反应容器的外层为加热保温层,可按照设定温度加热保温;反应釜的上部为转速可调的磁力搅拌器,通过电机带动搅拌棒将溶液与CO2充分混合均匀.实验时,CO2通过制冷系统液化后经柱塞泵高压注入反应釜内,达到设定压力.
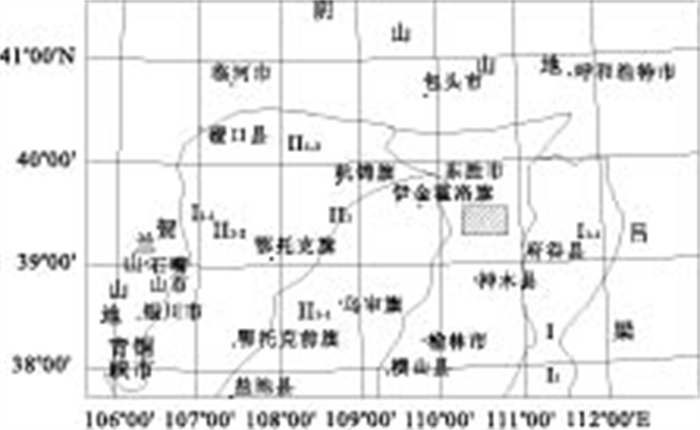
实验水样采自鄂尔多斯盆地榆林市大柳塔附近,具体位置如图 3阴影所示:
实验前,首先依据地下水标准检验方法的DZ/T0064-1993,对野外采集的鄂尔多斯盆地山西组地层水进行化学成分分析,结果见表 1.根据分析结果,采用分析纯试剂NaCl、KCl、MgCl2、CaCl2、NaHCO3人工配制溶液,用量如表 2所示,使之与鄂尔多斯盆地山西组地下水化学成分一致.
表 1 鄂尔多斯盆地山西组地下水化学成分Table Supplementary Table Chemical composition of Shanxi Formation groundwater in Ordos basin项目 ρB(mg·L-1) CB(mmol·L-1) pH 6.90 K+ 67.80 1.734 Na+ 2405.00 104.565 Ca2+ 888.50 22.168 Mg2+ 32.79 1.349 NH4+ <0.01 <0.001 Al3+ <0.02 — Cl- 5151.49 145.317 SO42- 29.10 0.303 HCO3- 642.01 10.521 表 2 人工合成鄂尔多斯盆地山西组地下水实际用量Table Supplementary Table Recipe for synthetic Shanxi Formation groundwater in Ordos basin试剂 级别 质量(g) NaCl 分析纯 5.5016 KCl 分析纯 0.1267 CaCl2 分析纯 2.4598 MgCl2 分析纯 0.1283 NaHCO3 分析纯 0.8838 实验开始时,在釜内加入2/3体积的水样,设定适当温度开始加热,同时用柱塞泵向高压釜内通入CO2直到釜内压力达到要求.打开磁力搅拌器,将CO2与水样充分混合.保持压力与温度不变,使整个系统运行24h,确保系统达到平衡状态.系统平衡后,用取样器从釜中取出一定量饱和CO2的水样.由于CO2溶解度会随温度压力的改变而发生变化,当水样从高压釜中被取出时,温度压力的降低,使溶解在水样中的CO2部分逸出.为减少CO2逸出造成的误差,采用图 2中所示取样器,该取样器由两部分组成:具塞刻度试管和气体取样袋,当其与高压釜连接时,整个体系为封闭状态.具塞刻度试管体积较小并且阻隔了样品与空气的接触,通过其取样前后的体积差,计算稀释因子F;气体取样袋则能够收集暂时从水样中逃逸的CO2.取样时先在取样器中加入一定量1mol/L的NaOH溶液,随后再从釜中取出适量饱和CO2的水样,此时NaOH将吸收所有CO2气体(包括暂时逃逸至取样袋中CO2)并转化为CO32-:
CO2(aq)+2NaOH→2Na++H2O+CO32−. (2) 取样时,记录取样前后的溶液体积分别为P、M,根据二者的差值计算稀释因子F:
F=MM−P. (3) 取样后,饱和CO2的水样与NaOH溶液发生化学反应,溶液中出现沉淀.根据溶液成分以及相关化学反应方程式:
MgCO3↔Mg2++CO32−, (4) Mg(OH)2↔Mg2++2OH−, (5) CaCO3↔Ca2++CO32−. (6) 依照室温下各难溶物的溶度积(华东理工大学化学系,2003)计算各难溶物析出沉淀时的最小浓度,以MgCO3的计算为例:
Ksp=[ Mg2+][CO32-]=[Mg2+]2=2.6×10-5.
由上式可得[Mg2+]=5.10×10-3mol/L.
即当溶液中[Mg2+]≥5.10×10-3mol/L时,MgCO3开始沉淀(以溶液中的CO32-离子足以使Mg2+沉淀为前提).已知原水样Mg2+浓度为1.35×10-3mol/L,小于5.10×10-3mol/L,故水样不生成MgCO3沉淀.稀释后,Mg2+浓度减小,沉淀反应不发生.
同理,经计算,Mg(OH)2开始沉淀时Mg2+的最小浓度为1.65×10-4mol/L,小于原水样中Mg2+浓度,故生成Mg(OH)2沉淀;CaCO3开始沉淀时Ca2+离子浓度为9.32×10-3mol/L,小于原水样中Ca2+离子浓度(0.2216mol/L),可以生成CaCO3沉淀,故取样后溶液中的沉淀为Mg(OH)2、CaCO3,且沉淀完全.
取出的样品经过滤后,采用双指示剂滴定法分析上清液碱度.为减少操作时水样与空气接触造成的误差,过滤及滴定过程需在5min内完成.用0.1mol/L的盐酸溶液作滴定剂来滴定1mL样品,滴定过程中分别用酚酞和甲基橙作为指示剂,达到两个滴定终点:pH≈8.0时达到第1个终点,此时溶液中OH-被中和,CO32-与H+反应生成HCO3-;pH≈4.3时达到第2个终点,此时溶液中所有的溶解态碳被完全中和,发生如下反应:
H++OH−=H2O, (7) H++CO32−=HCO3−, (8) HCO−3+H+=H2O+CO2. (9) 达到各滴定终点时消耗HCL的体积分别记为V1、V2,则溶液中OH-和CO32-的浓度分别为:
[OH−]=CHCl×(V1−V2)V0, (10) [CO2−3]=CHCl×V2V0. (11) 其中V0为滴定时所用样品体积,计算结果以mol/L为单位.
通过实验分析计算得到上清液中CO32-的浓度,这代表了溶解在水样中的一部分CO2,前文中提到取样后溶液中生成Mg(OH)2、CaCO3沉淀,可见,沉淀中包含了一部分溶解CO2.将计算得到的上清液中CO32-与Ca2+离子结合的CO32-相加,得到发生(2)反应的所有CO2,再通过(12)式计算,即可得到水样中CO2的溶解度S,计算结果以g/100g水样为单位,即:
S=[CO2−3]×F×440. (12) 2. 结果与讨论
实验测得了不同温度压力下CO2在水样中的溶解度,结果如表 3所示.
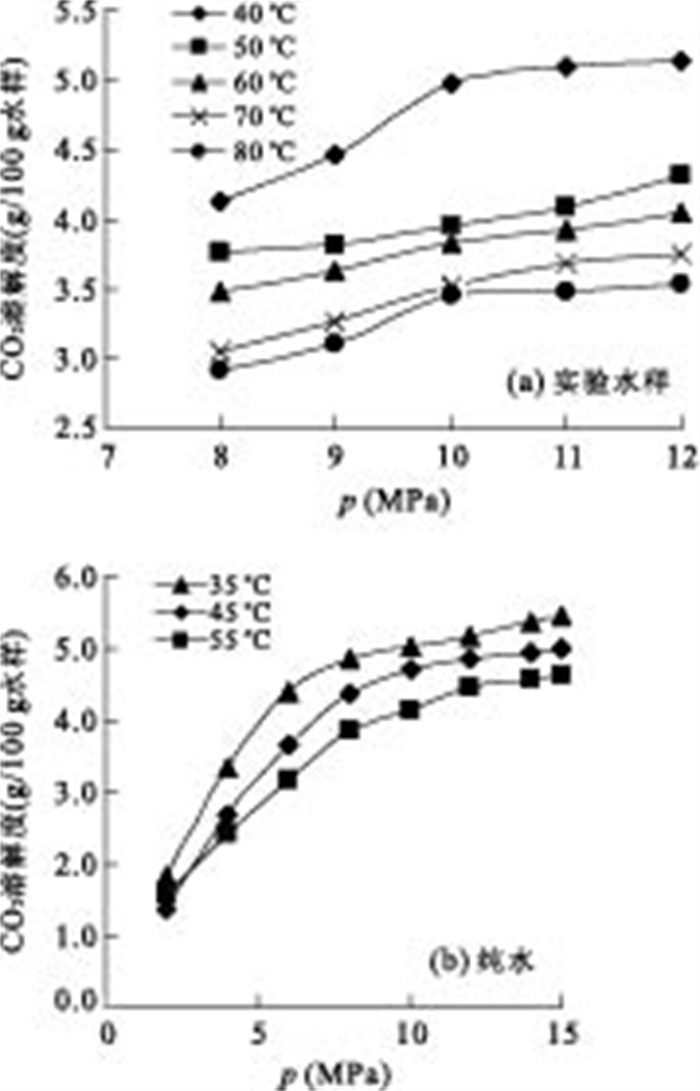
表 3 人工合成山西组水样CO2溶解度数据Table Supplementary Table Solubility of CO2 in synthetic Shanxi Formation waterp(MPa) t(℃) CO2溶解度(g/100g水样) 8 40 4.1325 8 50 3.7660 8 60 3.4672 8 70 2.8024 8 80 2.9234 9 40 4.4582 9 50 3.8240 9 60 3.6137 9 70 3.2732 9 80 3.1099 10 40 4.9088 10 50 3.8848 10 60 3.8328 10 70 3.6542 10 80 3.4597 11 40 5.1454 11 50 4.0946 11 60 3.9191 11 70 3.6859 11 80 3.3427 12 40 5.0948 12 50 4.3054 12 60 4.0520 12 70 3.6379 12 80 3.4382 从图 4a中可以看出,在所有实验温度范围内CO2在实验水样中的溶解度随压力的增加而增大.压力较低时,CO2溶解度随压力变化较大;而当压力较高时,CO2溶解度随压力的变化不明显.图 4b数据来自Liu et al.(2011)的文献数据,显示了不同温度下CO2在纯水的溶解度与压力变化的关系.比较图 4a与图 4b可知,CO2在实验水样中的溶解度随压力的变化与其在纯水中的变化相似.
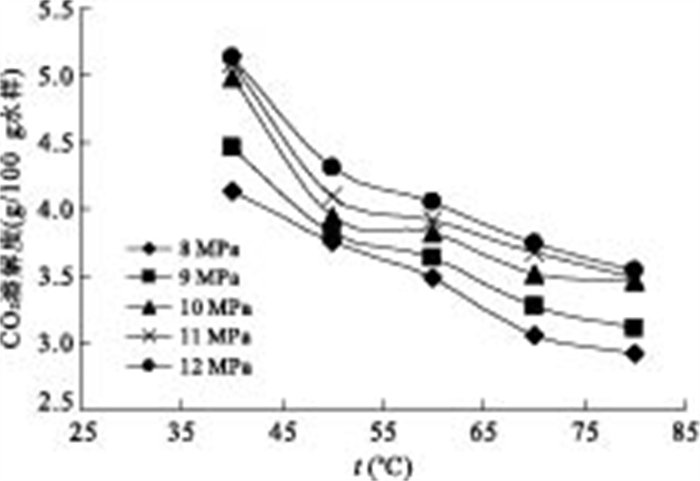
图 5描述了不同压力下CO2溶解度随温度的变化情况.从图中可以看出,在所有实验压力范围内,随温度的升高,CO2的溶解度呈下降趋势.
比较图 4a与图 5,同一压力下,溶解度随温度的变化较大,而同一温度下,溶解度随压力的变化较小,表明温度对CO2溶解度的影响要大于压力的影响.
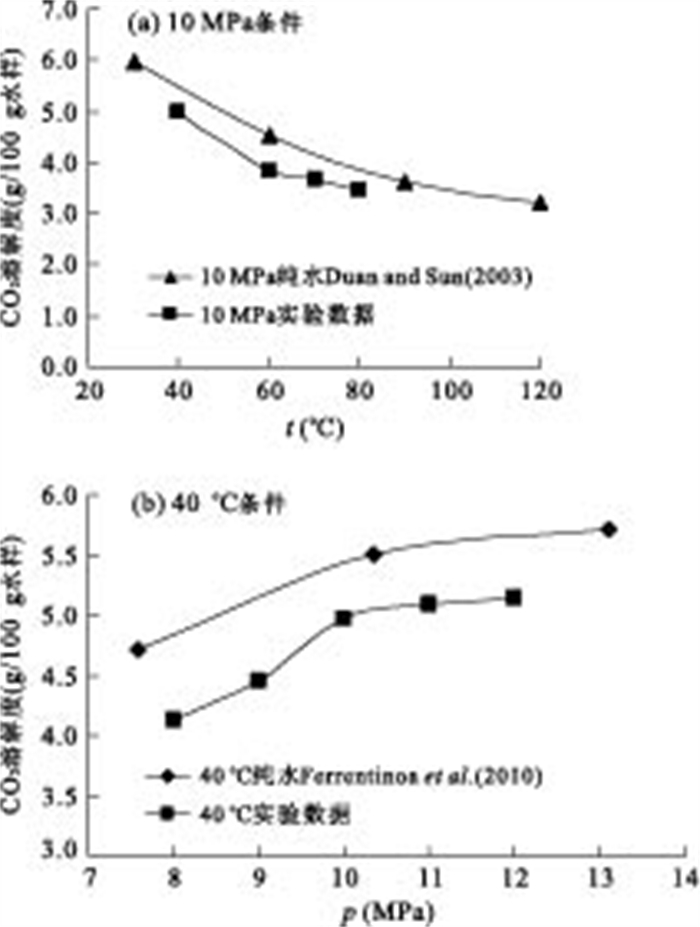
图 6a比较了10MPa压力条件下,CO2在纯水和实验水样中的溶解度差异.其中CO2在纯水中的溶解度数据来自Duan and Sun(2003)的研究.图 6b为40℃时CO2在纯水和实验水样中溶解度的比较,其中CO2在纯水中的溶解度数据来自Ferrentinoa et al.(2010)的研究.从图 6中可以看出,CO2在实验水样中的溶解度明显小于其在纯水中的溶解度.这种现象可以用盐析效应来解释,盐水中离子浓度较高,离子间相互作用较强,使得分子较难溶于水中,从而导致了CO2溶解度的降低.
3. 结语
本文以鄂尔多斯盆地山西组地下水为研究对象,通过实验测试得到了40~80℃、8~12MPa条件下,CO2在地层水中的溶解度,得到以下结论:(1)实验条件下CO2的溶解度为2.80~5.14g/100g水样;(2)对溶解度随温度压力变化的分析结果表明,CO2在该水样中的溶解度随压力升高而升高,随温度升高而降低,且温度对CO2溶解度的影响大于压力的影响;(3)对相同条件下CO2在实验水样中的溶解度与其在纯水中溶解度的差别比较分析表明,实验条件下,CO2在实验水样中的溶解度小于相同条件下CO2在纯水中的溶解度.
-
表 1 鄂尔多斯盆地山西组地下水化学成分
Table 1. Chemical composition of Shanxi Formation groundwater in Ordos basin
项目 ρB(mg·L-1) CB(mmol·L-1) pH 6.90 K+ 67.80 1.734 Na+ 2405.00 104.565 Ca2+ 888.50 22.168 Mg2+ 32.79 1.349 NH4+ <0.01 <0.001 Al3+ <0.02 — Cl- 5151.49 145.317 SO42- 29.10 0.303 HCO3- 642.01 10.521 表 2 人工合成鄂尔多斯盆地山西组地下水实际用量
Table 2. Recipe for synthetic Shanxi Formation groundwater in Ordos basin
试剂 级别 质量(g) NaCl 分析纯 5.5016 KCl 分析纯 0.1267 CaCl2 分析纯 2.4598 MgCl2 分析纯 0.1283 NaHCO3 分析纯 0.8838 表 3 人工合成山西组水样CO2溶解度数据
Table 3. Solubility of CO2 in synthetic Shanxi Formation water
p(MPa) t(℃) CO2溶解度(g/100g水样) 8 40 4.1325 8 50 3.7660 8 60 3.4672 8 70 2.8024 8 80 2.9234 9 40 4.4582 9 50 3.8240 9 60 3.6137 9 70 3.2732 9 80 3.1099 10 40 4.9088 10 50 3.8848 10 60 3.8328 10 70 3.6542 10 80 3.4597 11 40 5.1454 11 50 4.0946 11 60 3.9191 11 70 3.6859 11 80 3.3427 12 40 5.0948 12 50 4.3054 12 60 4.0520 12 70 3.6379 12 80 3.4382 -
Darwish, N.A., Hilal, N., 2010. A simple model for the prediction of CO2 solubility in H2O-NaCl system at geological sequestration conditions. Desalination, 260(1-3): 114-118. doi: 10.1016/j.desal.2010.04.056 Deparment of Chemistry, East China University of Science and Technology, et al., 2003. Analytical chemistry. Higher Education Press, Beijing, 425 (in Chinese). Duan, Z.H., Sun, R., 2003. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2 000 bar. Chem. Geol. , 193(3-4): 257-271. doi: 10.1016/S0009-2541(02)00263-2 Ellis, A.J., Golding, R.M., 1963. The solubility of carbon dioxide above 100 ℃ in water and in sodium chloride solutions. American Journal of Science, 261(1): 47-60. doi: 10.2475/ajs.261.1.47 Ferrentinoa, G., Barlettaa, D., Balaban, M.O., et al., 2010. Measurement and prediction of CO2 solubility in sodium phosphate monobasic solutions for food treatment with high pressure carbon dioxide. The Journal of Supercritical Fluids, 52(1): 142-150. doi: 10.1016/j.supflu.2009.10.005 Hou, G.C., Zhang, M.S., 2008. The survey and research on groundwater in Ordos basin. Geological Publishing House, Beijing, 85 (in Chinese). Li, X.C., Liu, Y.F., Bai, B., et al., 2006. Ranking and screening of CO2 saline aquifer storage zones in China. Chinese Journal of Rock Mechanics and Engineering, 25(5): 963-968 (in Chinese with English abstract). http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&cmd=prlinks&retmode=ref&id=21866545 Li, X.C., Ohsumia, T., Koide, H., et al., 2005. Near-future perspective of CO2 aquifer storage in Japan: site selection and capacity. Energy, 30(11-12): 2360-2369. doi: 10.1016/j.energy.2004.08.026 Liu, Y.H., Hou, M.Q., Yang, G.Y., et al., 2011. Solubility of CO2 in aqueous solutions of NaCl, KCl, CaCl2 and their mixed salts at different temperatures and pressures. The Journal of Supercritical Fluids, 56(2): 125-129. doi: 10.1016/j.supflu.2010.12.003 Ren, X.K., Cui, Y.J., Bu, X.P., et al., 2010. Analysis on CO2 storage potentiality in Ordos basin. Energy of China, 32(1): 29-32 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZGLN201001015.htm Shen, P.P., Liao, X.W., 2009. The technology of carbon dioxide stored in geological media and enhanced oil recovery. Petroleum Industry Press, Beijing, 39 (in Chinese). Teng, H., Yamasaki, A., 1998. Solubility of liquid CO2 in synthetic sea water at temperatures from 278 K to 293 K and pressures from 6.44 MPa to 29.49 MPa, and densities of the corresponding aquous solutions. J. Chem. Eng. Data. , 43(1): 2-5. doi: 10.1021/je9700737 Wiebe, R., Gaddy, V.L., 1939. The solubility in water of carbon dioxide at 50 ℃, 75 ℃ and 100 ℃, at pressures to 700 atmospheres. J. Am. Chem. Soc. , 61(2): 315-318. doi: 10.1021/ja01871a025 Wiebe, R., Gaddy, V.L., 1940. The solubility of carbon dioxide in water at various temperatures from 12 to 40 ℃ and at pressures to 500 atmospheres. J. Am. Chem. Soc. , 62(4): 815-817. doi: 10.1021/ja01861a033 Portier, S., Rochelle, C., 2005. Modeling CO2 solubility in pure water and NaCl-type waters from 0 to 300 ℃ and from 1 to 300 bar: application to the Utsira Formation at Sleipner. Chemical Geology, 217(3-4): 187-199. doi: 10.1016/j.chemgeo.2004.12.007 Zeng, R.S., Sun, S., Chen, D.Z., et al., 2004. Decrease carbon dioxide emission into the atmosphere-underground disposal of carbon dioxide. Bulletin of National Natural Science Foundation of China, 4: 196-200 (in Chinese with English abstract). http://d.wanfangdata.com.cn/periodical/zgkxjj200404002 华东理工大学化学系等, 2003. 分析化学. 北京: 高等教育出版社, 425. 侯光才, 张茂省, 2008. 鄂尔多斯盆地地下水勘查系统. 北京: 地质出版社, 85. 李小春, 刘延锋, 白冰, 等, 2006. 中国深部咸水含水层CO2储存优先区域选择. 岩石力学与工程学报, 25(5): 963-968. doi: 10.3321/j.issn:1000-6915.2006.05.015 任相坤, 崔永君, 步学朋, 等, 2010. 鄂尔多斯盆地CO2地质封存潜力分析. 中国能源, 32(1): 29-32. doi: 10.3969/j.issn.1003-2355.2010.01.006 沈平平, 廖新维, 2009. 二氧化碳地质埋存与提高石油采收率技术. 北京: 石油工业出版社, 39. 曾荣树, 孙枢, 陈代钊, 等, 2004. 减少二氧化碳向大气层的排放——二氧化碳地下储存研究. 中国科学基金, 4: 196-200. doi: 10.3969/j.issn.1000-8217.2004.04.002 -











 下载:
下载:





 下载:
下载: