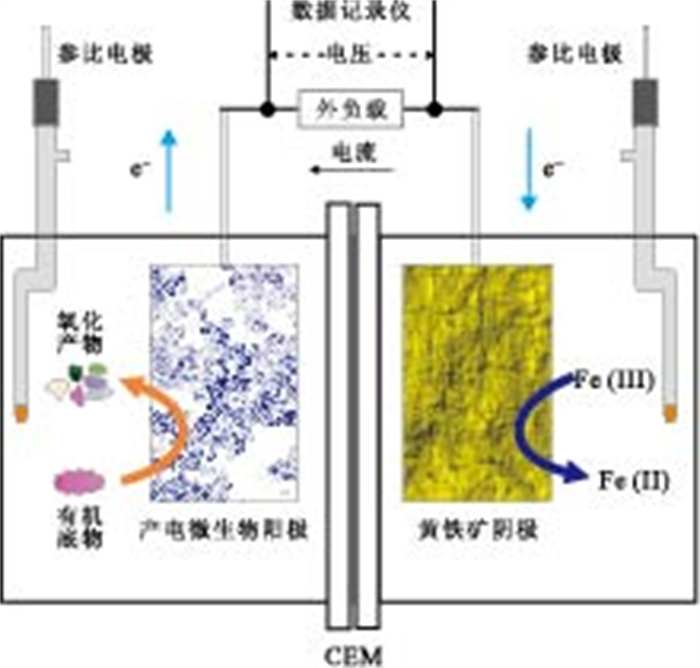
Electrochemical Study on Electron Transfer Process between Electricigens and Single Crystal Pyrite in a Dual-Chambered Equipment
-
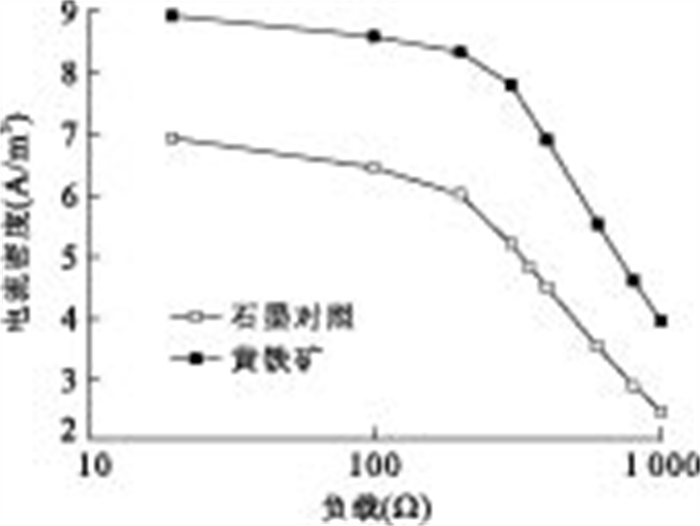
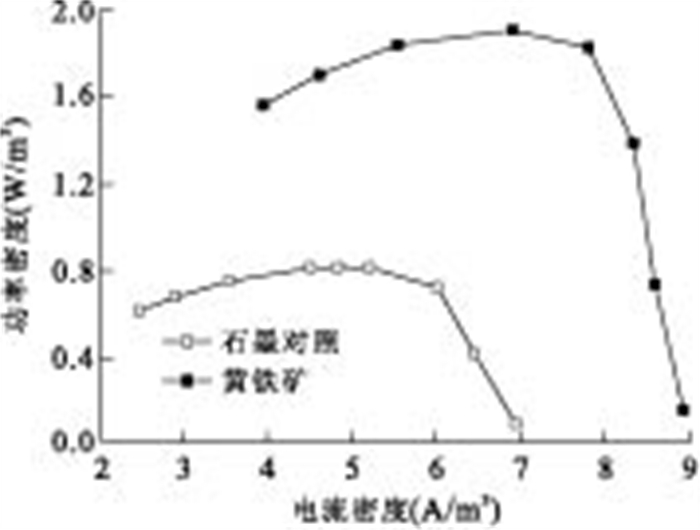
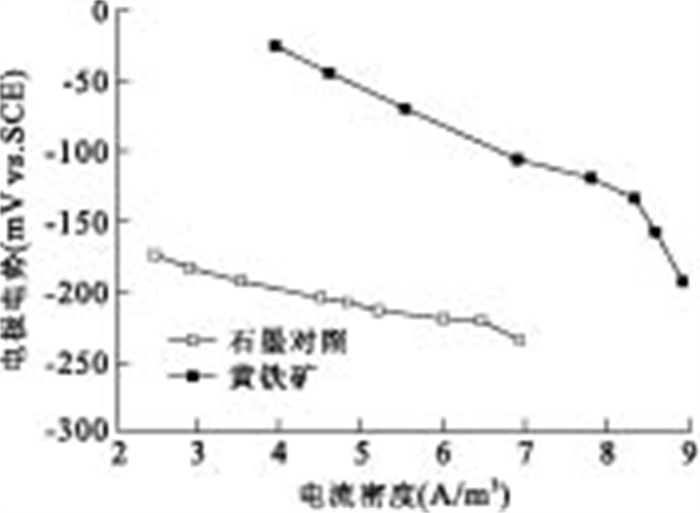
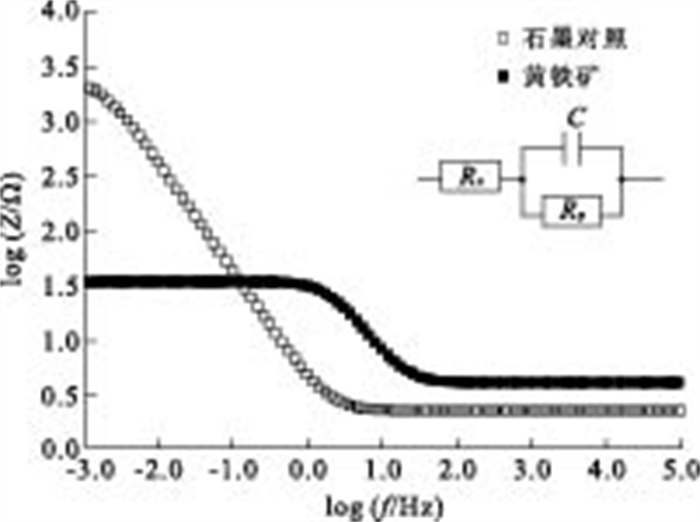
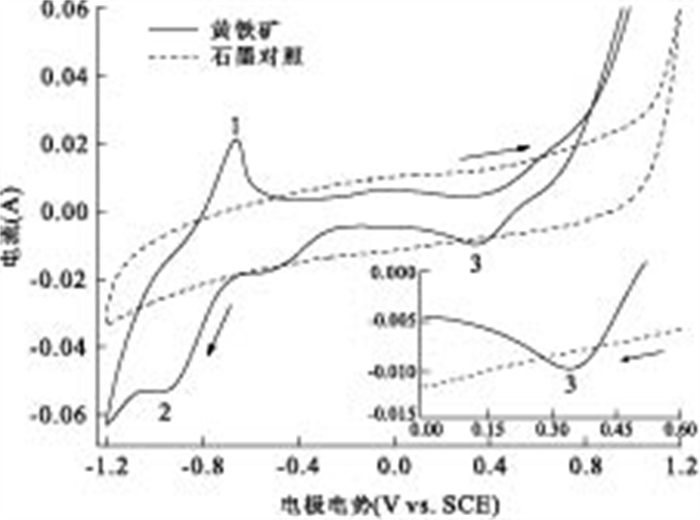
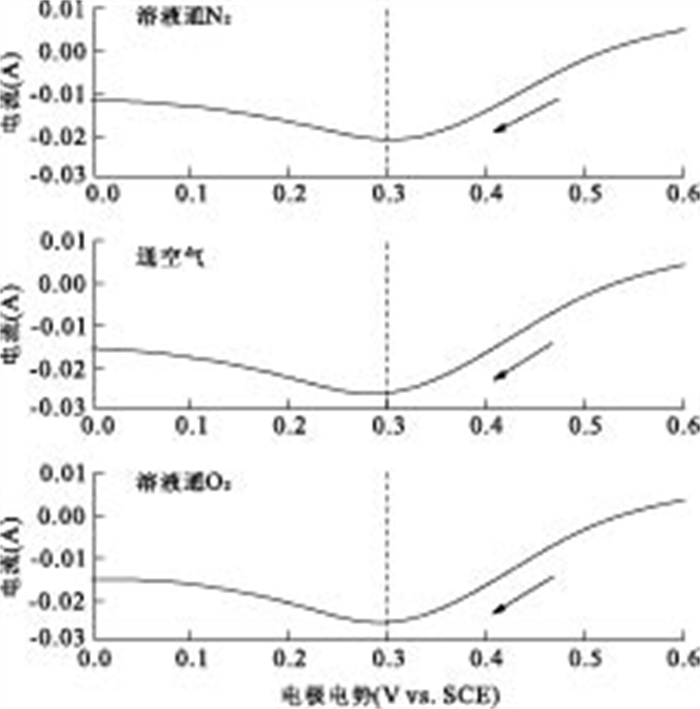
摘要: 通过构建产电微生物—黄铁矿双室体系, 应用电化学方法对以黄铁矿单晶电极作为产电微生物电子受体时, 两者间的电子转移过程进行表征和分析.结果显示, 与惰性石墨电极相比, 以黄铁矿单晶作为产电微生物电子受体时, 体系最大功率密度提升132.9%;电化学阻抗谱显示, 黄铁矿单晶电极极化电阻降低98.8%, 表现出优良的电化学反应特性, 表明产电微生物与黄铁矿单晶间具有良好的电子转移活性.籍由产电微生物对底物的氧化作用, 与黄铁矿单晶接受产电微生物电子在0.34 V(相对于饱和甘汞电极)处发生的还原反应, 构成了两者间完整的协同电子转移过程.Abstract: This research built up a dual-chambered electricigens-pyrite equipment. Using single crystal pyrite as electron acceptor of electricigens, and the electron transfer process was analyzed by electrochemical methods. Compared with graphite electrode, the maximum system power density increased by 132.9% and polarization resistance of EIS decreased by 98.8% with a single crystal pyrite electrode. The data show a favorable electron transfer activity between electricigens and single crystal pyrite. The electron transfer process is related to the two electrode reactions, of which one is microbial oxidation by electricigens, and the other is reduction at 0.34 V (vs. SCE) by single crystal pyrite as electron acceptor.
-
Key words:
- pyrite /
- electricigens /
- microbial reduction /
- electrochemisty /
- mineralogy /
- environmental engineering
-
表 1 黄铁矿电极/石墨对照EIS拟合参数
Table 1. Fit parameters for EIS of pyrite electrode and graphite as control
参数 黄铁矿 石墨对照 Rp(Ω) 30.35 2 523 Rs (Ω) 4.059 2.256 C(F) 0.002 475 0.037 26 -
Almeida, C.M.V.B., Giannetti, B.F., 2003. The electrochemical behavior of pyrite-pyrrhotite mixtures. Journal of Electroanalytical Chemistry, 553(30): 27-34. doi: 10.1016/S0022-0728(03)00254-7 Childers, S.E., Ciufo, S., Lovley, D.R., 2002. Geobacter metallireducens accesses insoluble Fe (III) oxide by chemotaxis. Nature, 416(6882): 767-769. doi: 10.1038/416767a Dean, J.A., 1991. Lange's handbook of chemistry (13rd Edition). Translated by Shang, J.F., Cao, S.J., Xin, W.M., et al. . Science Press, Beijing (in Chinese). Dong, H., 2010. Mineral-microbe interactions: a review. Frontiers of Earth Science in China, 4(2): 127-147. doi: 10.1007/s11707-010-0022-8 Evangelou, V.P.B., Zhang, Y.L., 1995. A review: pyrite oxidation mechanisms and acid mine drainage prevention. Critical Reviews in Environmental Science & Technology, 25(2): 141-199. doi: 10.1080/10643389509388477 Giorgi, L., Antolini, E., Pozio, A., et al., 1998. Influence of the PTFE content in the diffusion layer of low-Pt loading electrodes for polymer electrolyte fuel cells. Electrochimica Acta, 43(24): 3675-3680. doi: 10.1016/S0013-4686(98)00125-X Kim, H.J., Park, H.S., Hyun, M.S., et al., 2002. A mediator less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciense. Enzyme and Microbial Technology, 30(2): 145-152. doi: 10.1016/S0141-0229(01)00478-1 Liu, H., Logan, B.E., 2004. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environmental Science & Technology, 38(14): 4040-4046. doi: 10.1021/es0499344 Logan, B.E., Hamelers, B., Rozendal, R., et al., 2006. Microbial fuel cells: methodology and technology. Environmental Science & Technology, 40(17): 5181-5192. doi: 10.1021/es0605016 Manohar, A.K., Bretschger, O., Nealson, K.H., et al., 2008. The use of electrochemical impedance spectroscopy (EIS) in the evaluation of the electrochemical properties of a microbial fuel cell. Bioelectrochemistry, 72(2): 149-154. doi: 10.1016/j.bioelechem.2008.01.004 Marshall, C.W., May, H.D., 2009. Electrochemical evidence of direct electrode reduction by a thermophilic gram-positive bacterium, Thermincola ferriacetica. Energy & Environmental Science, 2(6): 699-705. doi: 10.1039/B823237G Myers, C.R., Nealson, K.H., 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science, 240(4857): 1319-1321. doi: 10.1126/science.240.4857.1319 Rabaey, K., Verstraete, W., 2005. Microbial fuel cells: novel biotechnology for energy generation. Trends in Biotechnology, 23(6): 291-298. doi: 10.1016/j.tibtech.2005.04.008 Roden, E.E., Urrutia, M.M., Mann, C.J., 2000. Bacterial reductive dissolution of crystalline Fe (III) oxide in continuous-flow column reactors. Applied and Environmental Microbiology, 66(3): 1062-1065. doi: 10.1128/AEM.66.3.1062-1065.2000 Schaetzle, O., Barrière, F., Baronian, K., 2008. Bacteria and yeasts as catalysts in microbial fuel cells: electron transfer from micro-organisms to electrodes for green electricity. Energy & Environmental Science, 1(6): 607-620. doi: 10.1039/B810642H Schippers, A., Jørgensen, B.B., 2002. Biogeochemistry of pyrite and iron sulfide oxidation in marine sediments. Geochimica et Cosmochimica Acta, 66(1): 85-92. doi: 10.1016/S0016-7037(01)00745-1 Schröder, U., Nießen, J., Scholz, F., 2003. A generation of microbial fuel cells with current outputs boosted by more than one order of magnitude. Angewandte Chemie, 115(25): 2986-2989. doi: 10.1002/ange.200350918 Tao, D.P., Richardson, P.E., Luttrell, G.H., et al., 2003. Electrochemical studies of pyrite oxidation and reduction using freshly-fractured electrodes and rotating ring-disc electrodes. Electrochimica Acta, 48(24): 3615-3623. doi: 10.1016/S0013-4686(03)00482-1 Zhao, F., Harnisch, F., Schröder, U., et al., 2006. Challenges and constraints of using oxygen cathodes in microbial fuel cells. Environmental Science & Technology, 40(17): 5193-5199. doi: 10.1021/es060332p Dean, J.A., 主编, 1991. 兰氏化学手册, 中文版(第十三版). 尚久方, 操时杰, 辛无名, 等译. 北京: 科学出版社. -









 下载:
下载: