Characteristics and Implications of Rare Earth Elements in High Arsenic Groundwater from the Datong Basin
-
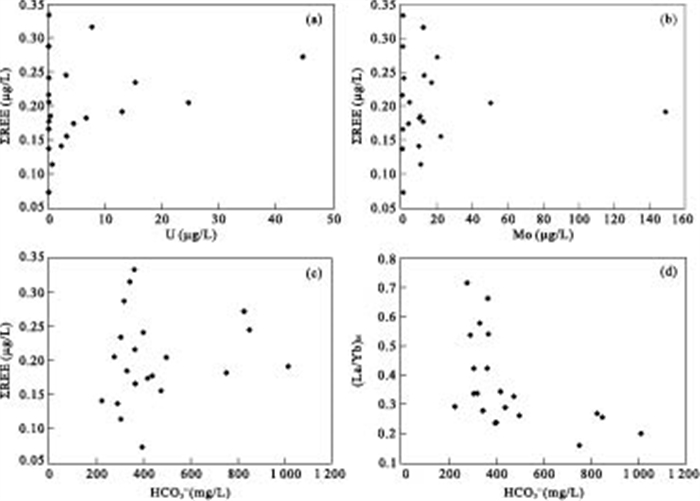
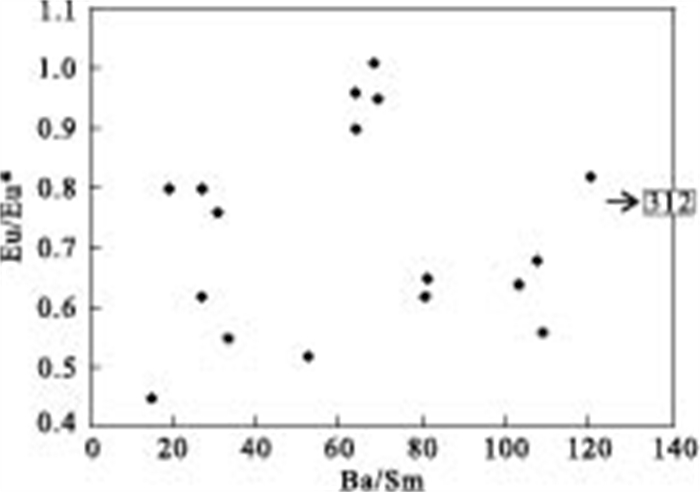
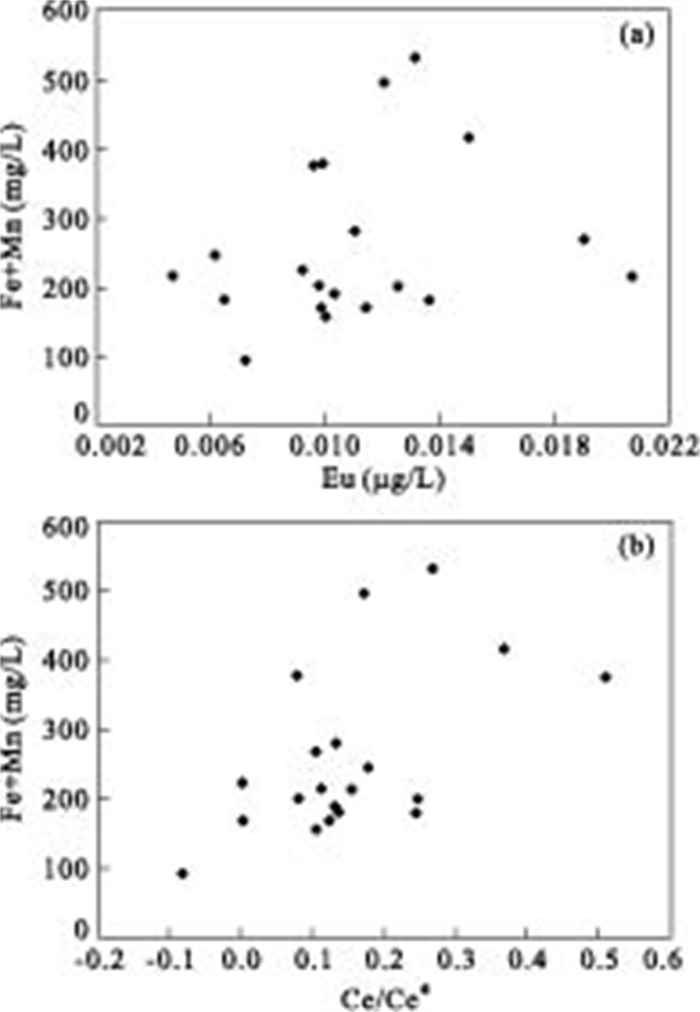
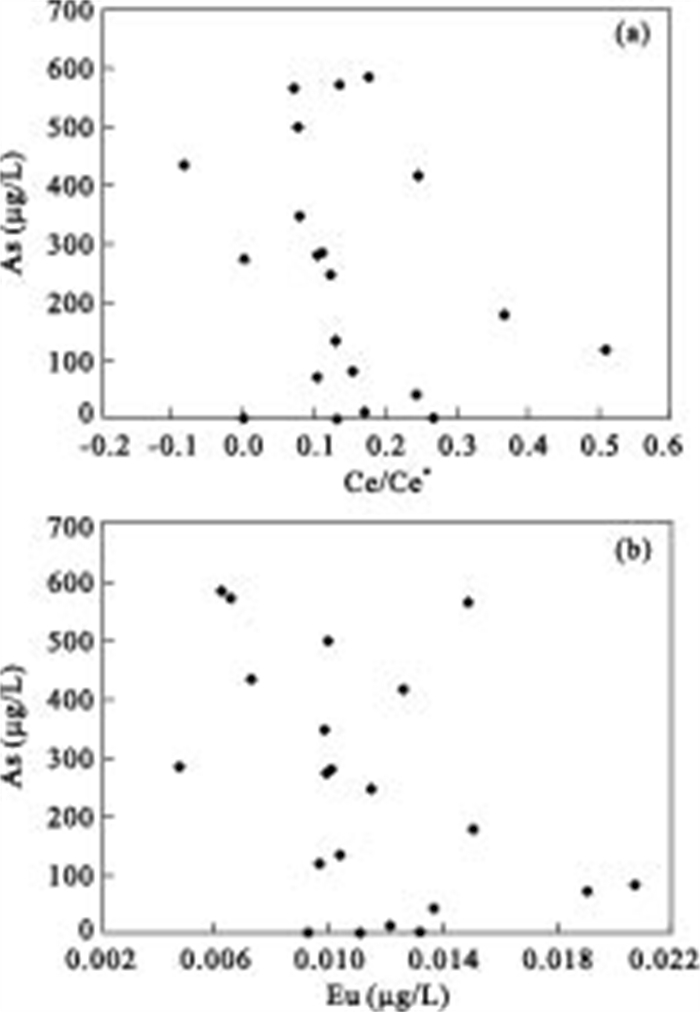
摘要: 对大同盆地典型高砷地下水开展了稀土元素地球化学研究.研究表明: 高砷地下水具有低∑REE含量及富集重稀土(HREEs)特征.地下水中低含量∑REE与含水层沉积物中Fe-Mn氧化物/氢氧化物对REEs的吸附有关.地下水中重稀土元素相对于轻稀土元素的富集可能是吸附作用和碳酸根离子同REEs发生络合作用的共同结果.采用平均大陆上地壳标准化的地下水稀土元素分布表现出显著的Ce及Eu正异常.地下水Ce/Ce*值及Eu含量与Fe+Mn具有显著相关性, 表明铁锰氧化物还原性溶解是控制Ce/Ce*值及Eu含量特征的主要因素.Ce/Ce*值及Eu含量与As浓度的关系表明, Ce异常及Eu含量特征能对地下水中As的富集进行有效指示.Abstract: In order to better understand the occurrence of high arsenic groundwater, rare earth elements (REEs) analyses were conducted for groundwater from the Datong basin. The results indicate that high arsenic groundwater usually has low ∑REE concentration and enriches in HREEs relative to LREEs. The low concentration of ∑REE in groundwater samples could be due to the scavenging of REEs onto the surface of solid phase Fe-Mn oxides/hydroxides within aquifer sediment. The enrichment of HREEs can be attributed to the combined result of complexation, and desorption and readsorption. The average up crust normalized REEs patterns clearly exhibit significant positive Ce and Eu anomalies in high arsenic groundwater. The observed good correlation between Ce/Ce* values and Eu and Fe+Mn could be related to the reductive dissolution of Fe and Mn oxides/hydroxides. The relationship between As and Ce/Ce* value and Eu suggests that Ce/Ce* value and Eu concentration are useful indicators of arsenic mobilization in groundwater system.
-
Key words:
- water pollution /
- arsenic /
- rare earth elements /
- redox reactions /
- hydrogeology /
- Datong basin
-
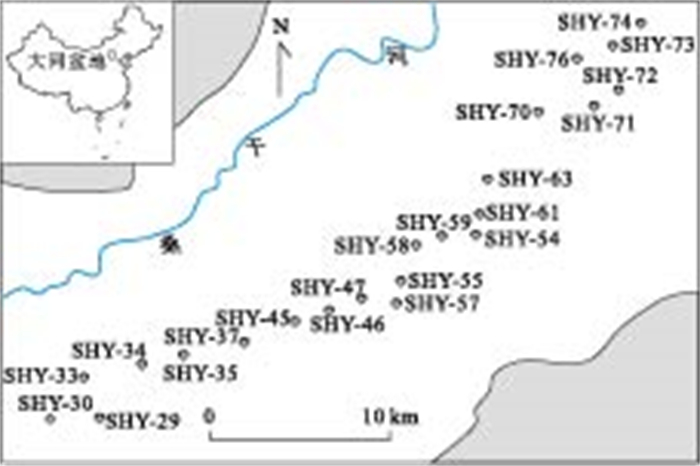
表 1 大同盆地高砷地下水水化学组成特征
Table 1. Chemistry of high arsenic groundwater from the Datong basin
样号 点位 pH Cl-(mg/L) SO42-(mg/L) HCO3-(mg/L) K(mg/L) Na(mg/L) Ca(mg/L) Mg(mg/L) Ba(mg/L) Mn(μg/L) Fe(μg/L) As(μg/L) Mo(μg/L) U(μg/L) SHY-29 112°39.77′E39°16.80′N 8.18 21.6 1.2 436 0.45 78.69 10.65 12.18 0.73 69.68 348.48 179.10 12.10 0.07 SHY-33 112°38.83′E39°17.89′N 7.75 100.9 204.7 495 0.72 136.53 33.65 56.91 1.44 53.03 229.52 1.55 50.21 24.72 SHY-34 112°41.37′E39°18.29′N 8.24 105.3 71.2 328 1.58 82.87 22.18 26.44 0.80 82.65 187.82 73.07 10.41 0.34 SHY-35 112°42.85′E39°18.62′N 8.13 44.4 49.6 473 0.71 88.37 16.43 29.93 89.44 158.25 585.10 22.04 3.19 SHY-37 112°45.32′E39°19.11′N 7.65 497.4 515.3 825 32.09 357.73 95.78 182.79 0.47 78.10 455.97 3.70 20.03 44.88 SHY-45 112°47.14′E39°19.74′N 8.05 44.9 24.8 416 0.43 79.80 12.12 25.86 0.91 36.49 146.99 572.00 3.80 4.41 SHY-46 112°48.46′E39°20.19′N 8.18 21.1 1.4 341 1.47 57.50 16.35 20.91 1.84 127.52 250.04 119.90 12.14 7.66 SHY-47 112°49.72′E39°20.57′N 8 19.8 1.9 303 0.20 40.21 13.88 21.66 0.61 83.12 134.35 284.90 10.66 0.66 SHY-55 112°51.22′E39°21.10′N 7.97 13.2 1.4 289 0.12 34.44 13.66 18.67 1.10 78.12 125.07 348.50 0.38 0.03 SHY-57 112°51.06′E39°20.48′N 7.64 6.6 7.8 222 0.90 17.82 34.37 6.35 1.05 279.85 218.23 14.01 9.64 2.25 SHY-58 112°51.94′E39°22.21′N 7.82 55.7 3.6 362 0.37 52.09 16.85 27.50 0.41 156.86 1 791.48 565.80 0.38 0.02 SHY-59 112°52.90′E39°22.45′N 7.73 658.4 613.7 750 1.89 521.16 27.47 91.54 0.32 198.3 182.03 499.70 9.85 6.64 SHY-60 112°54.13′E39°22.54′N 7.88 13.5 2.0 359 0.43 31.30 17.19 33.02 0.56 5.82 196.57 417.10 0.74 0.06 SHY-61 112°54.27′E39°23.20′N 7.93 12.0 <0.1 317 0.24 38.35 14.00 16.78 1.20 4.02 187.35 135.20 0.64 0.02 SHY-63 112°54.61′E39°24.21′N 7.8 492.3 516.6 847 3.55 416.29 32.07 122.59 0.58 14.48 202.25 83.48 12.57 3.09 SHY-70 112°56.30′E39°26.34′N 8.13 15.9 1.3 364 0.29 48.41 11.41 23.55 0.42 18.32 140.27 280.80 0.59 0.04 SHY-71 112°58.66′E39°26.48′N 8.1 14.4 2.9 303 0.42 31.83 22.98 19.45 0.25 2.86 222.97 2.21 16.77 15.3 SHY-72 112°59.40′E39°27.11′N 7.96 17.5 1.3 275 0.29 38.48 13.60 14.20 2.93 168.77 274.80 4.37 0.04 SHY-73 112°59.23′E39°28.42′N 8.34 17.9 1.5 398 0.22 58.88 13.39 22.27 0.82 3.02 168.14 247.80 1.15 0.06 SHY-74 113°0.24′E39°29.03′N 7.8 229.4 255.2 1 012 3.17 334.45 13.52 40.37 0.21 59.76 122.54 43.33 148.96 12.99 SHY-76 112°57.89′E39°28.04′N 8.02 16.7 1.6 393 0.25 61.37 11.91 21.58 4.17 91.09 434.90 0.87 0.02 表 2 大同盆地高砷地下水稀土元素含量特征(单位:μg/L)
Table 2. Concentration of REEs (μg/L) in high arsenic groundwater from the Datong basin
样号 La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu ∑REE Ce/Ce* Eu/Eu* (La/Yb)N SHY-29 0.017 6 0.080 1 0.003 5 0.022 1 0.010 5 0.015 1 0.005 5 0.001 6 0.005 9 0.001 9 0.006 9 0.001 0 0.004 5 0.000 8 0.177 0 0.37 0.95 0.29 SHY-33 0.027 0 0.073 7 0.005 7 0.035 1 0.017 7 0.011 1 0.006 3 0.001 6 0.007 3 0.002 3 0.006 0 0.001 5 0.007 6 0.001 5 0.204 5 0.13 0.65 0.26 SHY-34 0.023 5 0.070 0 0.006 7 0.026 0 0.012 4 0.019 1 0.007 5 0.000 9 0.004 6 0.002 2 0.006 9 0.000 9 0.003 0 0.001 1 0.184 8 0.10 0.96 0.58 SHY-35 0.018 8 0.063 7 0.004 9 0.021 4 0.014 5 0.006 2 0.003 5 0.000 7 0.005 0 0.001 5 0.008 6 0.001 0 0.004 2 0.001 1 0.155 2 0.18 0.54 0.33 SHY-37 0.028 8 0.120 8 0.007 7 0.035 9 0.015 1 0.013 2 0.007 3 0.002 7 0.010 9 0.003 3 0.015 6 0.002 1 0.007 9 0.000 9 0.272 2 0.27 0.76 0.27 SHY-45 0.025 8 0.077 5 0.006 5 0.020 7 0.008 4 0.006 5 0.004 7 0.001 4 0.005 5 0.001 1 0.008 8 0.001 1 0.005 5 0.000 5 0.173 9 0.14 0.68 0.34 SHY-46 0.027 2 0.196 7 0.007 1 0.024 3 0.005 9 0.009 7 0.009 4 0.001 1 0.005 0 0.001 3 0.018 9 0.000 9 0.007 2 0.001 2 0.315 9 0.51 0.76 0.28 SHY-47 0.015 6 0.046 8 0.004 3 0.013 2 0.005 6 0.004 8 0.006 5 0.000 9 0.004 0 0.001 2 0.005 1 0.000 8 0.003 4 0.001 4 0.113 7 0.11 0.56 0.34 SHY-55 0.018 2 0.045 5 0.004 1 0.021 7 0.010 6 0.009 9 0.010 7 0.000 8 0.003 3 0.001 0 0.007 1 0.001 1 0.002 5 0.000 4 0.136 7 0.08 0.64 0.54 SHY-57 0.018 2 0.054 5 0.003 9 0.013 7 0.008 7 0.012 1 0.008 7 0.000 8 0.007 7 0.000 6 0.005 7 0.000 9 0.004 6 0.000 7 0.140 8 0.17 0.82 0.29 SHY-58 0.032 2 0.087 7 0.008 9 0.023 6 0.014 8 0.014 9 0.007 8 0.001 0 0.007 1 0.001 7 0.010 6 0.001 2 0.003 6 0.001 0 0.216 1 0.07 0.80 0.66 SHY-59 0.020 3 0.058 3 0.006 0 0.023 6 0.011 8 0.010 0 0.010 7 0.001 5 0.012 7 0.005 4 0.007 8 0.002 2 0.009 4 0.002 2 0.1819 0.08 0.62 0.16 SHY-60 0.037 0 0.150 2 0.010 2 0.034 8 0.016 7 0.012 6 0.016 7 0.002 4 0.010 1 0.002 1 0.032 2 0.001 2 0.006 4 0.001 1 0.333 7 0.25 0.55 0.42 SHY-61 0.040 8 0.118 4 0.009 8 0.045 3 0.014 8 0.010 4 0.008 8 0.002 0 0.006 6 0.002 3 0.017 8 0.000 5 0.008 9 0.001 1 0.287 5 0.13 0.62 0.34 SHY-63 0.028 3 0.096 8 0.008 4 0.027 5 0.008 4 0.020 7 0.010 4 0.001 9 0.012 0 0.005 1 0.014 2 0.001 6 0.008 2 0.001 4 0.245 0 0.15 1.01 0.25 SHY-70 0.026 6 0.067 4 0.005 5 0.023 9 0.006 5 0.010 1 0.005 6 0.002 0 0.004 9 0.001 1 0.007 4 0.000 9 0.003 6 0.000 4 0.165 8 0.10 0.90 0.54 SHY-71 0.040 3 0.081 0 0.008 4 0.035 3 0.016 4 0.009 3 0.014 8 0.000 9 0.006 4 0.001 7 0.009 5 0.001 5 0.007 0 0.001 9 0.234 4 0.00 0.45 0.42 SHY-72 0.032 1 0.073 7 0.008 7 0.028 7 0.009 8 0.009 9 0.014 9 0.000 9 0.010 3 0.001 8 0.008 7 0.001 2 0.003 3 0.001 5 0.205 3 0.00 0.57 0.72 SHY-73 0.032 9 0.080 2 0.005 6 0.038 6 0.015 5 0.011 5 0.016 8 0.002 1 0.011 1 0.002 3 0.012 5 0.000 7 0.010 0.001 1 0.241 1 0.12 0.52 0.24 SHY-74 0.020 6 0.077 2 0.004 9 0.022 2 0.010 5 0.013 7 0.009 8 0.001 3 0.006 5 0.002 9 0.012 4 0.001 1 0.007 6 0.000 7 0.191 3 0.24 0.80 0.20 SHY-76 0.011 3 0.017 0 0.001 9 0.011 1 0.002 8 0.007 3 0.004 9 0.000 7 0.005 3 0.001 1 0.004 1 0.000 6 0.003 5 0.000 9 0.072 4 -0.08 0.94 0.23 注:下标N表示上地壳标准化值;(La/Yb)N=(LaN/YbN);Ce/Ce*=log[CeN/(LaN+PrN)];Eu/Eu*=log[EuN/(SmN+GdN)]. -
Appelo, C.A.J., Weiden, M.J.J.V.D., Tournassat, C., et al., 2002. Surface complexation of ferrous iron and carbonate on ferrihydrite and the mobilization of Arsenic. Environmental Science & Technology, 36(14): 3096-3103. doi: 10.1021/es010130n Banks, D., Hall, G., Reimann, C., et al., 1999. Distribution of rare earth elements in crystalline bedrock groundwaters: oslo and Bergen regions, Norway. Applied Geochemistry, 14(1): 27-39. doi: 10.1016/S0883-2927(98)00037-7 Bau, M., 1999. Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanide tetrad effect. Geochimica et Cosmochimica Acta, 63(1): 67-77. doi: 10.1016/S0016-7037(99)00014-9 Bau, M., Usui, A., Pracejus, B., et al., 1998. Geochemistry of low-temperature water-rock interaaction: evidence from natural waters, andesite, and iron-oxyhydroxide precipitates at Nishiki-numa iron-spring, Hokkaido, Japan. Chemical Geology, 151(1-4): 293-307. doi: 10.1016/S0009-2541(98)00086-2 Braun, J.J., Viers, J., Dupré, B., et al., 1998. Solid/liquid REE fractionation in the lateritic system of Goyoum, East Cameroon: the implication for the present dynamics of the soil covers of the humid tropical regions. Geochimica et Cosmochimica Acta, 62(1): 273-299. doi: 10.1016/S0016-7037(97)00344X Dia, A., Gruau, G., Olivié-Lauquet, G., et al., 2000. The distribution of rare earth elements in groundwaters: assessing the role of source-rock composition, redox changes and colloidal particles. Geochimica et Cosmochimica Acta, 64(24): 4131-4151. doi: 10.1016/S0016-7037(00)004944-1 Dulski, P., 1994. Interferences of oxide, hydroxide and chloride analyte species in the determination of rare earth elements in geological samples by inductively coupled plasma-mass spectrometry. Fresenius' Journal of Analytical Chemistry, 350(4-5): 194-203. doi: 10.1007/BF00322470 Gorby, Y.A., Lovley, D.R., 1992. Enzymatic uranium precipitation. Environmental Science & Technology, 26(1): 205-207. doi: 10.1021/es00025a026 Gui, H.R., Sun, L.H., 2011. Rare earth element geochemical characteristics of the deep underground water from Renlou coal mine, northern Anhui Province. Journal of China Coal Society, 36(2): 210-216 (in Chinese with English abstract). http://www.ingentaconnect.com/content/jccs/jccs/2011/00000036/00000002/art00006 Guo, H.M., Yang, S.Z., Tang, X.H., et al., 2008. Groundwater geochemistry and its implications for arsenic mobilization in shallow aquifers of the Hetao basin, Inner Mongolia. Science of the Total Environment, 393(1): 131-144. doi: 10.1016/j.scitotenv.2007.12.025 Guo, H.M., Zhang, B., Li, Y., et al., 2010. Concentrations and patterns of rare earth elements in high arsenic groundwaters from the Hetao plain, Inner Mongolia. Earth Science Frontiers, 17(6): 59-66 (in Chinese with English abstract). http://www.ingentaconnect.com/content/el/18725791/2010/00000017/00000006/art00008 Guo, H.M., Zhang, B., Wang, G.C., et al., 2010. Geochemical controls on arsenic and rare earth elements approximately along a groundwater flow path in the shallow aquifer of the Hetao basin, Inner Mongolia. Chemical Geology, 270(1-4): 117-125. doi: 10.1016/j.chemgeo.2009.11.010 Hsi, C.K.D., Langmuir, D., 1985. Adsorption of uranyl onto ferric oxyhydroxides: application of the surface complexation sitebinding model. Geochimica et Cosmochimica Acta, 49(9): 1931-1941. doi: 10.1016/0016-7037(85)90088-2 Jiang, S.Y., Zhao, H.X., Chen, Y.Q., et al., 2007. Trace and rare earth element geochemistry of phosphate nodules from the lower Cambrian black shale sequence in the Mufu Mountain of Nanjing, Jiangsu Province, China. Chemical Geology, 244(3-4): 584-604. doi: 10.1016/j.chemgeo.2007.07.010 Johannesson, K.H., Stetzenbach, K.J., Hodge, V.F., 1997. Rare earth elements as geochemical tracers of regional groundwater mixing. Geochimica et Cosmochimica Acta, 61(1): 3605-3618. doi: 10.1016/S0016-7037(97)00177-4 Johannesson, K.H., Zhou, X.P., Guo, C.X., et al., 2000. Origin of rare earth element signatures in groundwaters of circumneutral pH from southern Nevada and eastern California, USA. Chemical Geology, 164(3-4): 239-257. doi: 10.1016/S0009-2541(99)00152-7 Lee, S.G., Lee, D.H., Kim, Y., et al., 2003. Rare earth elements as indicators of groundwater environment changes in a fractured rock system: evidence from fracture-filling calcite. Applied Geochemistry, 18(1): 135-143. doi: 10.1016/S0883.2927(02)00071-9 Lowers, H.A., Breit, G.N., Foster, A.L., et al., 2007. Arsenic incorporation into authigenic pyrite, Bengal basin sediment, Bangladesh. Geochimica et Cosmochimica Acta, 71(11): 2699-2717. doi: 10.1016/j.gca.2007.03.022 Luo, Y.L., Jiang, P.A., Yu, Y.H., et al., 2006. Investigation and assessment on arsenic pollution of soil and groungwater in Kuitun No. 123 State Farm. Arid Land Geography, 29(5): 705-709 (in Chinese with English abstract). http://www.cnki.com.cn/Article/CJFDTotal-GHDL200605017.htm Nath, B., Jean, J.S., Lee, M.K., et al., 2008. Geochemistry of high arsenic groundwater in Chia-Nan plain, southwestern Taiwan: possible sources and reactive transport of arsenic. Journal of Contaminant Hydrology, 99(1-4): 85-96. doi: 10.1016/j.jconhyd.2008.04.005 Nickson, R.T., McArthur, J.M., Ravenscroft, P., et al., 2000. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Applied Geochemistry, 15(4): 403-413. doi: 10.1016/S0883-2927(99)00086-4 Nordstrom, D.K., 2002. Worldwide occurrences of arsenic in ground water. Science, 296(5576): 2143-2145. doi: 10.1126/science.1072375 Ohta, A., Kawabe, I., 2001. REE(III) adsorption onto Mn dioxide (δ-MnO2) and Fe oxyhydroxide: Ce (III) oxidation by δ-MnO2. Geochimica et Cosmochimica Acta, 65(5): 695-703. doi: 10.1016/S0016-7037(00)00578-0 Oremland, R.S., Stolz, J.F., 2005. Arsenic, microbes and contaminated aquifers. Trends in Microbiology, 13(2): 45-49. doi: 10.1016/j.tim.2004.12.002 Polya, D.A., Gault, A.G., Diebe, N., et al., 2005. Arsenic hazard in shallow Cambodian groundwaters. Mineralogical Magazine, 69(5): 807-823. doi: 10.1180/0026461056950290 Postma, D., Larsen, F., Minh Hue, N.T., et al., 2007. Arsenic in groundwater of the Red River floodplain, Vietnam: controlling geochemical processes and reactive transport modeling. Geochimica et Cosmochimica Acta, 71(21): 5054-5071. doi: 10.1016/j.gca.2007.08.020 Romero, L., Alonso, H., Campano, P., et al., 2003. Arsenic enrichment in waters and sediments of the Rio Loa (Second Region, Chile). Applied Geochemistry, 18(9): 1399-1416. doi: 10.1016/S0883-2927(03)00059-3 Sholkovitz, E.R., Landing, W.M., Lewis, B.L., 1994. Ocean particle chemistry: the fractionation of rare earth elements between suspended particles and seawater. Geochimica et Cosmochimica Acta, 58(6): 1567-1579. doi: 10.1016/0016-7037(94)90559-2 Smedley, P.L., Kinniburgh, D.G., 2002. A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17(5): 517-568. doi: 10.1016/S0883-2927(02)00018-5 Smedley, P.L., Nicolli, H.B., Macdonald, D.M.J., et al., 2002. Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Applied Geochemistry, 17(3): 259-284. doi: 10.1016/S0883-2927(01)00082-8 Tang, J., Johannesson, K.H., 2006. Controls on the geochemistry of rare earth elements along a groundwater flow path in the Carrizo Sand aquifer, Texas, USA. Chemical Geology, 225(1-2): 156-171. doi: 10.1016/j.chemgeo.2005.09.007 Tossell, J.A., 2005. Calculating the partitioning of the isotopes of Mo between oxidic and sulfidic species in aqueous solution. Geochimica et Cosmochimica Acta, 69(12): 2981-2993. doi: 10.1016/j.gca.2005.01.016 Tweed, S.O., Weaver, T.R., Cartwright, I., et al., 2006. Behavior of rare earth elements in groundwater during flow and mixing in fractured rock aquifers: an example from the Dandenong Ranges, Southeast Australia. Chemical Geology, 234(3-4): 291-307. doi: 10.1016/j.chemgeo.2006.05.006 Verplanck, P.L., Mueller, S.H., Goldfarb, R.J., et al., 2008. Geochemical controls of elevated arsenic concentrations in groundwater, Ester Dome, Fairbanks district, Alaska. Chemical Geology, 255(1-2): 160-172. doi: 10.1016/j.chemgeo.2008.06.020 Wang, Y.X., Shvartsev, S.L., Su, C.L., 2009. Genesis of arsenic/fluoride-enriched soda water: a case study at Datong, northern China. Applied Geochemistry, 24(4): 641-649. doi: 10.1016/j.apgeochem.2008.12.015 Wood, S.A., 1990. The aqueous geochemistry of the rare-earth elements and yttrium: 1. review of available low-temperature data for inorganic complexes and the inorganic REE speciation of natural waters. Chemical Geology, 82: 159-186. doi: 10.1016/0009-2541(90)90080-Q Xie, X.J., Wang, Y.X., Su, C.L., et al., 2008. Arsenic mobilization in shallow aquifers of Datong basin: hydrochemical and mineralogical evidences. Journal of Geochemical Exploration, 98(3): 107-115. doi: 10.1016/j.gexplo.2008.01.002 桂和荣, 孙林华, 2011. 皖北任楼煤矿深层地下水稀土元素地球化学特征. 煤炭学报, 36(2): 210-216. https://www.cnki.com.cn/Article/CJFDTOTAL-MTXB201102009.htm 郭华明, 张波, 李媛, 等, 2010. 内蒙古河套平原高砷地下水中稀土元素含量及分异特征. 地学前缘, 17(6): 59-66. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY201006007.htm 罗艳丽, 蒋平安, 余艳华, 等, 2006. 土壤及地下水砷污染现状调查与评价——以新疆奎屯123团为例. 干旱区地理, 29(5): 705-709. doi: 10.3321/j.issn:1000-6060.2006.05.015 -








 下载:
下载: