Hydrogeochemical Characteristics and Genesis of Luohe Iron Deposit
-
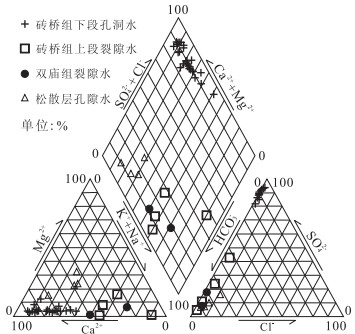
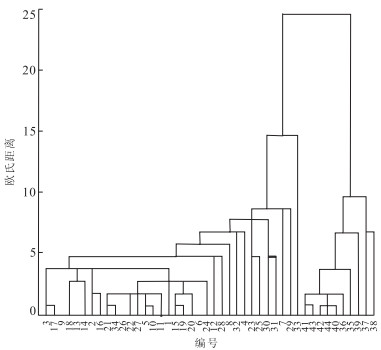
摘要: 作为庐枞盆地较早开展勘探工作的罗河铁矿, 矿区揭露的地下水可分为潜水和承压水2类, 潜水表现为弱碱性HCO3--Ca+·Mg2+型水; 区域构造和凝灰岩阻隔了潜水和承压水的水力联系, 承压水以SO42--Ca2+型微咸温水为主, 水化学成分不受外界因素变化影响.离子比例系数和相关性分析说明承压水中主要水化学反应包括硫酸盐矿物溶解和阳离子交换.PHREEQC反向模拟SO42--Ca2+型承压水的成因主要是砖桥组下段次生石英岩中大量硬石膏、石英和水云母的原位溶解; 与此同时, 地下水中沉淀生成了方解石和绿泥石, 石膏溶解的Ca2+离子吸附交换粘土矿物中的Na+离子, 少量黄铁矿还发生了氧化还原反应.分析结果验证了罗河铁矿深部地下水相对封闭, 补给有限, 以静储量为主.Abstract: As one well-explored region of Luzong Mesozoic volcanic basin, the groundwater revealed in Luohe iron deposit can be divided into two types, namely, unconfined and confined groundwater. The unconfined groundwater is alkalescent HCO3--Ca+·Mg2+ water, while the confined groundwater is the SO42--Ca2+ brackish warm water due to the fact that their hydrochemical compositions are almost not affected by external changes because of the water blocking properties of regional structure and volcanic tuff. Ion proportionality coefficients and correlation analyses illustrate that major hydrochemical reactions in confined groundwater are sulfate mineral solution and cation exchange. PHREEQC inverse simulation shows that the cause of SO42--Ca2+ confined groundwater is the numerous anhydrite, quartz and hydromica solution in site, at the same time, some calcite and chlorite precipitate as the secondary minerals. Meanwhile, Ca2+that was dissolved from anhydrite absorbs and exchanges with Na+ in clay mineral, and few pyrites have redox reaction. The analysis verifies the hydrogeological condition of relative closed, limited recharge and static reserves of deep groundwater in Luohe iron deposit.
-
Key words:
- geochemistry /
- inverse simulation /
- cluster analysis /
- ore deposits /
- Luohe iron deposit
-
表 1 罗河铁矿地下水水化学测试结果
Table 1. Groundwater hydrochemical test data of Luohe iron deposit
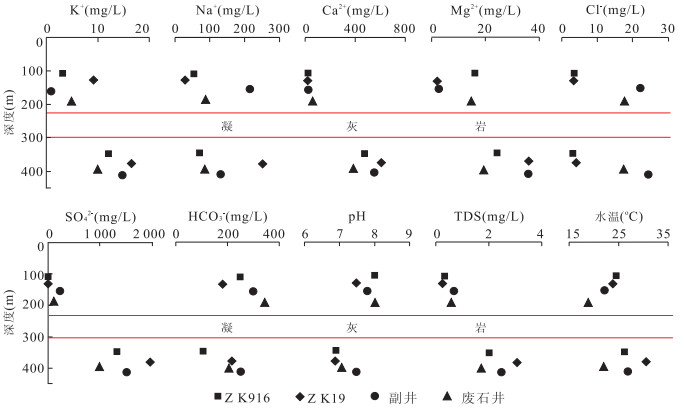
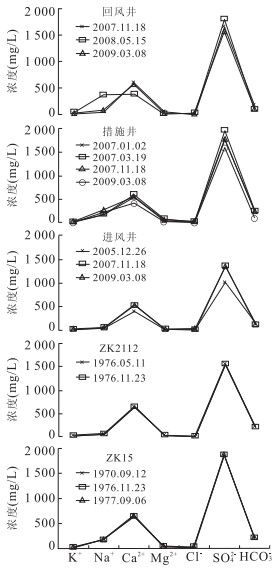
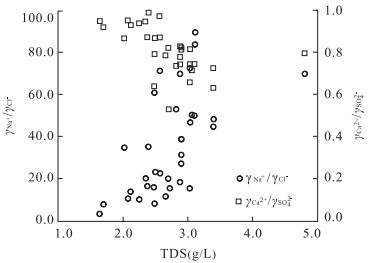
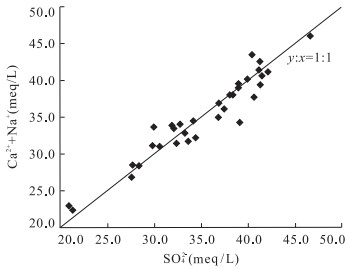
编号 取样点 类型 K+ Na+ Ca2+ Mg2+ Cl- SO42- HCO3- pH TDS 1 ZK1312 矿层水 21.500 155.000 541.480 22.370 10.280 1 626.780 119.600 7.20 2.60 2 ZK12 砖桥组承压水 16.270 287.800 598.800 38.670 9.220 1 978.490 244.690 7.80 3.39 3 ZK22 17.920 225.210 614.430 39.160 7.450 1 987.130 233.090 6.40 2.88 4 ZK14 19.580 381.340 588.370 40.690 13.120 2 233.620 218.450 6.40 3.39 5 ZK211 9.300 42.290 634.900 27.000 2.800 1 435.480 221.530 6.70 2.35 6 ZK09 14.110 151.970 522.310 33.410 20.210 1 594.560 201.040 7.20 2.66 7 ZK24 2.800 89.600 607.750 102.320 7.520 1 875.000 179.740 6.70 2.49 8 ZK25 24.200 198.000 617.020 40.760 4.380 1 866.450 218.940 6.90 4.80 9 ZK28 17.300 168.000 542.280 33.200 4.250 1 639.740 201.360 6.90 3.04 10 ZK2112 13.400 50.000 630.700 28.680 2.190 1 529.490 198.920 6.80 2.50 11 ZK2112 12.200 49.000 636.870 23.220 1.060 1 570.580 190.990 7.20 2.39 12 ZK916 12.000 70.000 476.150 24.190 3.100 1 321.000 113.500 6.90 2.02 13 ZK19 16.600 246.380 612.020 36.240 4.250 1 973.550 217.830 6.90 3.11 14 ZK26 8.300 240.380 593.180 33.320 5.320 1 915.200 214.180 7.30 2.88 15 ZK08 16.600 158.000 623.010 36.820 7.800 1 837.920 216.620 7.60 2.90 16 ZK03 13.280 253.730 602.000 42.200 7.800 2 021.760 201.970 7.80 3.06 17 ZK15 17.760 231.440 667.810 38.670 9.220 1 940.630 230.040 6.40 2.90 18 ZK15 21.000 164.000 635.050 37.400 3.490 1 866.450 209.300 7.00 3.03 19 ZK15 16.200 150.000 628.450 23.330 8.510 1 822.560 218.450 7.50 2.89 20 ZK132 19.500 151.320 605.210 30.700 11.730 1 768.700 193.470 7.30 2.70 21 ZK1712 14.940 51.930 573.950 21.280 7.800 1 468.200 155.600 7.30 2.25 22 ZK176 18.800 65.000 564.530 18.970 4.960 1 430.300 191.760 7.50 2.35 23 废石井 10.000 86.400 383.390 19.440 17.340 1 001.090 206.370 7.10 1.70 24 副井 15.000 130.000 556.430 35.730 24.280 1 537.400 250.980 7.50 2.49 25 进风井 7.500 51.560 402.780 24.420 24.280 1 018.960 153.400 7.50 1.64 26 进风井 21.040 47.070 526.300 34.360 5.270 1 358.290 109.360 7.50 2.12 27 进风井 7.860 47.400 527.000 16.500 6.930 1 329.000 130.100 7.50 2.08 28 措施井 25.980 180.990 540.080 59.540 5.270 1 765.100 220.610 7.90 2.82 29 措施井 17.730 170.000 604.600 88.450 5.270 1 948.100 245.120 6.90 3.10 30 措施井 29.100 281.900 542.400 44.180 28.130 1 979.220 109.360 7.50 3.03 31 措施井 20.090 233.000 430.000 36.440 22.540 1 612.000 89.090 7.50 2.48 32 回风井 12.730 51.410 599.100 63.810 3.520 1 649.350 175.350 7.60 2.57 33 回风井 48.750 376.900 393.900 16.650 37.840 1 798.200 93.820 7.44 2.72 34 回风井 15.780 74.000 564.000 26.100 6.930 1 553.000 110.500 7.40 2.38 35 ZK916 砖桥组潜水 3.200 55.400 23.450 15.810 3.300 12.970 246.620 8.00 0.33 36 ZK19 9.200 30.000 28.660 0.020 3.190 22.260 177.570 7.50 0.25 37 副井 1.000 218.000 21.620 3.170 22.230 243.130 303.090 7.80 0.70 38 废石井 5.000 91.200 65.650 14.920 17.340 114.410 345.800 8.00 0.60 39 QK1 双庙组潜水 5.400 91.250 30.060 9.240 5.320 61.440 288.010 7.30 0.49 40 QK3 5.600 30.300 32.700 0.370 7.700 14.610 178.790 7.80 0.18 41 包山 松散层潜水 3.800 9.800 20.840 10.210 8.860 12.010 109.220 7.40 0.13 42 李院子 3.000 8.200 51.300 10.820 7.800 12.970 199.500 7.60 0.21 43 曹庄 0.300 8.800 15.630 6.930 10.280 13.920 67.120 7.50 0.12 44 白棵树 0.400 13.700 24.050 17.270 10.280 12.000 170.850 7.50 0.17 注:离子浓度单位为mg/L,TDS(固体溶解总量)单位为g/L. 表 2 砖桥组地下水水化学离子比例系数
Table 2. Proportionality coefficients of groundwater hydrochemical ion of Zhuanqiao Formation
地下水类型 潜水 承压水 离子比例系数 最大值 最小值 平均值 最大值 最小值 平均值 γCa2+/γNa+ 1.10 0.11 0.64 17.26 1.20 6.47 γMg2+/γCa2+ 1.12 0.13 0.47 0.28 0.05 0.11 γNa+/γCl- 24.51 8.12 15.57 89.48 3.28 34.15 γCa2+/γSO42- 4.34 0.21 2.25 1.06 0.53 0.82 γCa2+/γHCO3- 0.58 0.22 0.39 15.57 5.67 9.78 γSO42-/γCl- 8.09 2.91 5.26 1 095.83 31.04 224.47 γHCO3-/γCl- 43.49 7.93 23.86 104.86 1.44 20.38 γSO42-/γHCO3- 1.02 0.07 0.42 24.36 6.16 12.29 表 3 砖桥组承压水相关矩阵系数之间的比值
Table 3. Pearson coefficient of correlation matrix of confined groundwater of Zhuanqiao Formation
水化学参数 K+ Na+ Ca2+ Mg2+ Cl- SO42- HCO3- pH TDS TDS 1.000 K+ 1.000 0.189 0.075 -0.027 0.027 0.221 -0.218 0.142 0.244 Na+ 1.000 -0.008 0.009 0.425 0.762 0.330 -0.081 0.593 Ca2+ 1.000 0.013 -0.661 0.582 0.593 -0.308 0.537 Mg2+ 1.000 -0.084 0.046 0.251 0.203 0.126 Cl- 1.000 -0.072 -0.368 0.241 -0.144 SO42- 1.000 0.376 -0.206 0.801 HCO3- 1.000 -0.282 0.431 pH 1.000 -0.202 注:以上离子比例系数为mEq/L. 表 4 水文地球化学模拟点水化学测试结果
Table 4. Chemical test data of hydrogeochemical simulation points
水样编号 水温 pH K+ Na+ Ca2+ Mg2+ Cl- SO42- HCO3- SiO2 Fe3+ 35 24.5 8.0 3.20 52.40 23.45 15.81 3.30 12.97 246.62 26.40 0.08 12 26.3 6.9 12.00 70.00 476.15 24.19 3.10 1 321.00 113.50 30.00 0.20 注:水温单位为℃,离子浓度单位mg/L. 表 5 模拟点主要离子组分存在形式及其含量(mol/kg)
Table 5. Major iron form and contents of simulation points
组分 存在形式 初始水 终态水 组分 存在形式 初始水 终态水 C(4) HCO3- 4.77×10-3 2.14×10-3 Cl- Cl- 9.31×10-5 8.76×10-5 CO2 9.93×10-5 4.97×10-4 K K+ 8.18×10-5 2.96×10-4 CaHCO3+ 2.36×10-5 1.12×10-4 Mg2+ Mg2+ 6.09×10-4 6.26×10-4 MgHCO3+ 2.46×10-5 8.13×10-6 MgSO42- 8.72×10-6 3.63×10-4 CO32- 2.82×10-5 1.37×10-6 MgHCO3+ 2.46×10-5 8.13×10-6 CaCO3 1.33×10-5 4.73×10-6 Na+ Na+ 2.26×10-3 2.98×10-3 Ca2+ Ca2+ 5.42×10-4 8.00×10-3 NaHCO3 5.13×10-6 2.50×10-6 CaSO42- 6.64×10-6 3.79×10-3 NaSO4- 9.66×10-7 7.11×10-5 CaHCO3+ 2.36×10-5 1.12×10-6 S(6) SO42- 1.19×10-4 9.54×10-3 CaCO3 1.33×10-5 4.73×10-6 CaSO42- 6.64×10-6 3.79×10-3 Fe Fe(OH)3 1.21×10-6 1.69×10-6 MgSO42- 8.72×10-6 3.63×10-4 Fe(OH)2+ 1.05×10-7 1.88×10-6 Si H4SiO4 4.33×10-4 5.00×10-4 表 6 砖桥组次生石英岩矿物饱和指数(SI)计算
Table 6. Saturation index calculation of minerals of secondary quartzite of Zhuanqiao Formation
饱和指数(SI) 初始水 终态水 硬石膏 -3.12 -0.36 方解石 0.38 -0.07 CO2(g) -2.54 -1.82 白云石 0.94 -1.09 O2(g) -35.36 -39.15 石英 0.62 0.66 表 7 砖桥组承压水水-岩相互作用矿物相的转移量
Table 7. Transfer amount of mineral phases in water-rock interaction of confined groundwater of Zhuanqiao Formation
矿物 硬石膏 石英 绿泥石 方解石 转移量 1.33×10-2 4.20×10-5 -4.33×10-4 -4.06×10-3 矿物 水云母 黄铁矿 Ca2+交换 Na+交换 转移量 3.76×10-4 2.16×10-6 -3.35×10-4 6.71×10-4 注:单位为mol/kg(H2O),矿物质量转移为正数表示溶解,负数表示沉淀;对Ca2+-Na+交换而言,正值表示Ca2+的降低以及溶液中Na+的升高;负值表示Ca2+的升高以及溶液中Na+的降低. -
Aiuppa, A., Avino, R., Brusca, L., et al., 2006. Mineral Control of Arsenic Content in Thermal Waters from Volcano-Hosted Hydrothermal Systems: Insights from Island of Ischiaand Phlegrean Fields (Campanian Volcanic Province, Italy). Chemical Geology, 229(4): 313-330. doi: 10.1016/j.chemgeo.2005.11.004 Casentini, B., Pettine, M., Millero, F.J., 2010. Release of Arsenic from Volcanic Rocks through Interactions with Inorganic Anions and Organic Ligands. Aquatic Geochemistry, 16(3): 373-393. doi: 10.1007/s10498-010-9090-3 Chu, X.L., Chen, J.S., Wang, S.X., 1984. Sulfur Isotopic Temperatures and Their Significance of Luohe Iron Deposit in Anhui Province. Geochimica, 3(4): 350-356 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-DQHX198404006.htm Chu, X.L., Chen, J.S., Wang, S.X., 1986. Study on Fractionation Mechanism of Sulfur Isotope and Physicochemical Conditions of Alteration and Pre Formation in Luohe Iron Deposit, Anhui. Scientia Geologica Sinica, 3: 276-289 (in Chinese with English abstract). http://www.researchgate.net/publication/279762114_Chinese_with_English_abstractStudy_on_fractionation_mechanism_of_sulfur_isotope_and_physicochemical_conditions_of_alteration_and_ore_formation_in_Louhe_iron_deposit_Anhui Cuoco, E., Verrengia, G., Francesco, S.D., et al., 2010. Hydrogeochemistry of Roccamonfina Volcano (Southern Italy). Environment Earth Science, 61(3): 525-538. doi: 10.1007/s12665-009-0363-3 Han, D.M., 2007. Analysis of Groundwater Flow System and Modeling of Hydrogeochemical Evolution in Xinzhou Basin, China (Dissertation). China University of Geosciences, Wuhan, 88-91 (in Chinese with English abstract). Huang, Q.T., 1984. A Brief Discussion on Geological Characteristics of the Luohe Iron Deposit in Anhui Province. Mineral Deposits, 3(4): 9-19 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-KCDZ198404001.htm Huang, W.B., Zhang, R.H., Hu, S.M., 2010. Chemistry Kinetic Experiment of Water-Rock Interaction in the Luohe Iron Deposit, Lujiang-Zongyang Basin, Anhui Province, China. Geological Bulletin of China, 29(10): 1579-1585 (in Chinese with English abstract). http://www.zhangqiaokeyan.com/academic-journal-cn_geological-bulletin-china_thesis/0201252285648.html Iwagami, S., Maki, T., Yuichi, O., et al., 2010. Role of Bedrock Groundwater in the Rainfall-Runoff Process in a Small Headwater Catchment Underlain by Volcanic Rock. Hydrological Processes, 24(19): 2771-2783. doi: 10.1002/hyp.7690 Jiang, M.R., 2001. Geological Characteristics and Metallogenic Regularity of Nihe Iron Deposit in Lujiang, Anhui Province (Dissertation). China University of Geosciences, Wuhan, 63-64 (in Chinese with English abstract). Li, X.L., 1992. On the Mineralization Model of "Three Sources-Heat, Water and Uranium"—Take the Model of Uranium Mineralization in Discharge Areas (Depressurization Areas) of Fossil Geothermal Systems in Mesozoic-Cenozoic Volcanic Magmatic Active Areas of Southeastern China. Journal of East China Geological Institute, 15(2): 101-112, 129 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-HDDZ199202000.htm Liu, W.S., 1993. Discussion on Groundwater Quality Evolution Mechanism in Hebei Plain to the South of Beijing and Tianjin. Site Investigation Science and Technology, 3: 36-39 (in Chinese with English abstract). Ren, Q.J., Wang, D.Z., Xu, Z.W., et al., 1993. Formation and Development of the Mesozoic Lujiang-Zongyang Volcanic-Structural Depression in Anhui Province and Their Relation to Mineralization. Acta Geologica Sinica, 67(2): 131-145 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-DZXE199302003.htm Shao, F., 2005. Low-Temperature Hot-Water in Xiangshan Orefield and Its Relation with Uranium Mineralization. Earth Science—Journal of China University of Geosciences, 30(2): 206-210, 240 (in Chinese with English abstract). http://www.researchgate.net/publication/289240147_Low-temperature_hot-water_in_Xiangshan_orefield_and_its_relation_with_uranium_mineralization Shen, Z.L., Zhu, W.H., Zhong, Z.S., 1993. Basis of Hydrogeochemistry. Geological Publishing House, Beijing, 87 (in Chinese). Su, Q., Yu, H.J., Xu, X.Y., et al., 2011. Hydrochemical Characteristics of Underground Brine in Littoral Plain South of Laizhou Bay. Advances in Marine Science, 29(2): 163-169 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-HBHH201102006.htm Wei, Y.N., Li, P.Y., Qian, H., et al., 2010. Research and Application of Hydro-Geochemical Simulation. Journal of Water Resources & Water Engineering, 21(1): 58-61 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-XBSZ201001013.htm Wu, Q.H., Wang, H.T., Zhang, C.S., et al., 1983. Sulfur Isotope Studies of the Dabaozhuang and Luohe Iron Deposits with an Approach to Their Genesis. Mineral Deposits, 2(4): 26-34 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-KCDZ198304003.htm Yan, R.S., 1984. Problems on the Geotemperature of the Luohe Iron Deposit. Geological Review, 30(5): 489-494 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-DZLP198405010.htm Zhang, G.X., Deng, W., He, Y., et al., 2006. Hydrochemical Characteristics and Evolution Laws of Groundwater in Songnen Plain, Northeast China. Advances in Water Science, 17(1): 20-28 (in Chinese with English abstract). Zhang, J.Z., 1990. The Metallogenic Characteristics and Prospecting Direction of the Mesozoic Volcano-Hydrothermal Gold Deposits in Eastern China. Mineral Resources and Geology, 4(2): 17-20, 24 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-KCYD199002003.htm Zhao, W.G., Wu, M.A., Zhang, Y.Y., et al., 2011. Geological Characteristics and Genesis of the Nihe Fe-S Deposit, Lujiang County, Anhui Province. Acta Geologica Sinica, 85(5): 789-801 (in Chinese with English abstract). http://www.researchgate.net/publication/284632358_Geological_characteristics_and_genesis_of_the_Nihe_Fe-S_deposit_Lujiang_County_Anhui_Province Zhou, H.F., Tan, H.B., Zhang, X.Y., et al., 2011. Recharge Source, Hydrochemical Characteristics and Formation Mechanism of Groundwater in Nantong, Jiangsu Province. Geochimica, 40(6): 566-576 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-DQHX201106007.htm Zhou, T.F., Fan, Y., Yuan, F., et al., 2008. The Study on Chronology and Its Significance of Volcanic Rocks in the Lujiang-Zongyang Basin, Anhui Province. Science in China (Series D), 38(11): 1342-1353 (in Chinese). Zhou, W.B., Li, X.L., 1995. Analysis of Paleohydrogeology for the Mineralization in Xiangshan Uranium Ore-Field. Geological Journal of Universities, 1(1): 101-108 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-GXDX501.011.htm 储雪蕾, 陈锦石, 王守信, 1984. 安徽罗河铁矿的硫同位素温度及意义. 地球化学, 3(4): 350-356. doi: 10.3321/j.issn:0379-1726.1984.04.007 储雪蕾, 陈锦石, 王守信, 1986. 罗河铁矿的硫同位素分馏机制和矿床形成的物理化学条件的研究. 地质科学, 3: 276-289. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKX198603009.htm 韩冬梅, 2007. 忻州盆地第四系地下水流动系统分析与水化学场演化模拟(博士学位论文). 武汉: 中国地质大学, 88-91. 黄清涛, 1984. 论罗河铁矿床地质特征及矿床成因. 矿床地质, 3(4): 9-19. https://www.cnki.com.cn/Article/CJFDTOTAL-KCDZ198404001.htm 黄文斌, 张荣华, 胡书敏, 2010. 安徽庐枞盆地罗河铁矿水岩反应化学动力学实验. 地质通报, 29(10): 1579-1585. doi: 10.3969/j.issn.1671-2552.2010.10.024 江满容, 2001. 安徽庐江泥河铁矿矿床地质特征及成矿规律研究(硕士学位论文). 武汉: 中国地质大学, 63-64. 李学礼, 1992. 论热源、水源、矿(铀)源三源成矿问题——以中国东南部中新生代火山-岩浆活动区古水热系统排泄区(减压区)铀成矿模式为例. 华东地质学院学报, 15(2): 101-112, 129. https://www.cnki.com.cn/Article/CJFDTOTAL-HDDZ199202000.htm 刘文生, 1993. 京津以南河北平原地下水水质演化机制的探讨. 勘察科学技术, 3: 36-39. https://www.cnki.com.cn/Article/CJFDTOTAL-KCKX199903007.htm 任启江, 王德滋, 徐兆文, 等, 1993. 安徽庐枞火山-构造洼地的形成、演化及成矿. 地质学报, 67(2): 131-145. https://www.cnki.com.cn/Article/CJFDTOTAL-DZXE199302003.htm 邵飞, 2005. 相山矿田低温热水及其与铀矿化关系. 地球科学——中国地质大学学报, 30(2): 206-210, 240. https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX20050200C.htm 沈照理, 朱宛华, 钟佐燊, 1993. 水文地球化学基础. 北京: 地质出版社, 87. 苏乔, 于洪军, 徐兴勇, 等, 2011. 莱州湾南岸滨海平原地下卤水水化学特征. 海洋科学进展, 29(2): 163-169. doi: 10.3969/j.issn.1671-6647.2011.02.005 魏亚妮, 李培月, 钱会, 等, 2010. 水文地球化学模拟研究与应用. 水资源与水工程学报, 21(1): 58-61. https://www.cnki.com.cn/Article/CJFDTOTAL-XBSZ201001013.htm 巫全淮, 王华田, 章纯荪, 等, 1983. 大鲍庄和罗河铁矿区硫同位素特征及其成因的探讨. 矿床地质, 4: 26-34. https://www.cnki.com.cn/Article/CJFDTOTAL-KCDZ198304003.htm 阎如璲, 1984. 罗河铁矿之地温问题. 地质论评, 30(5): 489-494. doi: 10.3321/j.issn:0371-5736.1984.05.011 章光新, 邓伟, 何岩, 等, 2006. 中国东北松嫩平原地下水水化学特征与演变规律. 水科学进展, 17(1): 20-28. doi: 10.3321/j.issn:1001-6791.2006.01.004 张甲忠, 1990. 我国东部中生代火山热液金矿床成矿特征和找矿方向. 矿产与地质, 4(2): 17-20, 24. https://www.cnki.com.cn/Article/CJFDTOTAL-KCYD199002003.htm 赵文广, 吴明安, 张宜勇, 等, 2011. 安徽省庐江县泥河铁矿床地质特征及成因初步分析. 地质学报, 85(5): 789-801. 周慧芳, 谭红兵, 张西营, 等, 2011. 江苏南通地下水补给源、水化学特征及形成机理. 地球化学, 40(6): 566-576. https://www.cnki.com.cn/Article/CJFDTOTAL-DQHX201106007.htm 周涛发, 范裕, 袁峰, 等, 2008. 安徽庐枞(庐江-枞阳)盆地火山岩的年代学及其意义. 中国科学(D辑), 38(11): 1342-1353. doi: 10.3321/j.issn:1006-9267.2008.11.002 周文斌, 李学礼, 1995. 相山铀矿田成矿古水文地质分析. 高校地质学报, 1(1): 101-108. https://www.cnki.com.cn/Article/CJFDTOTAL-GXDX501.011.htm -









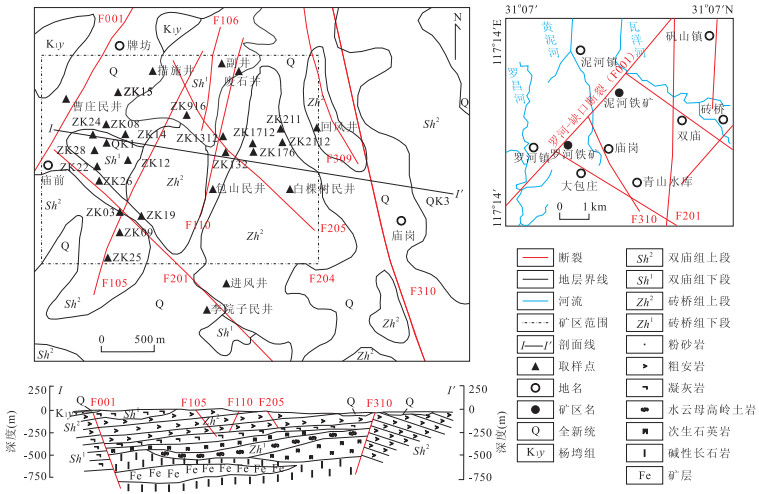
 下载:
下载: