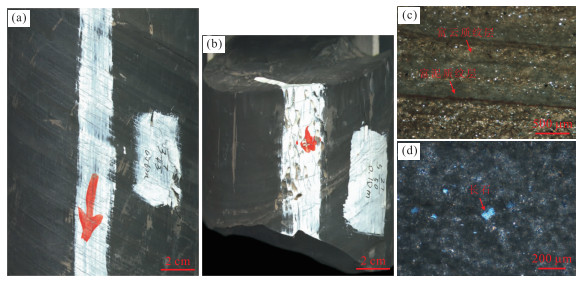
Major and Trace Elements Geochemistry and Geological Implications of Dolomite-Bearing Mudstones in Lower Part of Shahejie Formation in Tanggu Area, Eastern China
-
摘要: 细粒泥岩记录并反映着源区及沉积区的重要地质信息.塘沽地区沙河街组下部发育一套湖相含云质泥岩, 为了解该地区的构造背景、沉积背景、风化程度以及物源属性, 对25块含云质泥岩的地球化学特征进行了全面的分析.经"去白云石"调整后, 各类元素地球地化参数及图解反映出了一致的地质信息.其中, 高∑REE值(154.0×10-6~219.3×10-6)、中等强度的负铕异常(0.64~0.73)以及包括K2O/Na2O-SiO2, SiO2/Al2O3-K2O/Na2O和判别函数几类双变量图解均指示塘沽地区具有主动大陆边缘(安第斯型)的构造背景; SiO2-(K2O+Na2O+Al2O3)图解指示岩石沉积期气候干旱, 相当B(200.17~313.21)指示沉积水体为咸水类型, 近似黄铁矿矿化度(approximate degree of pyritization, 简称DOPapx)(0.02~0.38)、自生铀(0.14~1.22)及U/Th(0.17~0.44)指示水体具备常氧属性; 化学蚀变指数(chemical index of alteration, 简称CIA)(51.26~65.74), 长石蚀变指数(plagioclase index of alteration, 简称PIA)(51.65~78.06), 修正成分变异指数(modified index of composition variation, 简称ICVm)(0.67~1.24)及A-CN-K图解反映了母岩经受了弱-中等强度的风化程度; 判别函数图解、K-Rb双变量判别图解、A-CN-K图解、Al2O3/TiO2指标(23.37~28.58)、与上地壳(upper continental crust, 简称UCC)相似的稀土配分模式及负铕异常则共同确定长英质岩石为含云质泥岩的母岩来源.在上述背景条件的约束下, 研究表明塘沽地区沙河街组含云质泥岩的物质来源应主要由燕山褶皱带提供.Abstract: Fine mudstone can record and reflect geological aspects of source and sedimentary area. A set of lacustrine dolomite-bearing mudstones is developed in Lower Shahejie Formation of Tanggu area. To understand its tectonic setting, depositional setting, weathering and provenance, the geochemical characteristics of 25 rock samples were analyzed thoroughly. With dolomite-free modification, different indices and diagrams reveal identical geological information. Active continental margin setting (Andean-type) is revealed by the combination of high ∑REE(154.0×10-6-219.3×10-6), moderate negative Eu anomaly (0.64-0.73) and tectonic setting discriminant diagram (K2O/Na2O-SiO2, SiO2/Al2O3-K2O/Na2O, and discriminant function bivariate diagram). SiO2-(K2O+Na2O+Al2O3) bivariate discriminant diagram, equivalent B(200.17-313.21) and approximate degree of pyritization (DOPapx) (0.02~0.38), authigenic U(0.14-1.22), U/Th(0.17-0.44) indicate arid, saline and oxic depositional condition respectively. Chemical index of alteration (CIA)(51.26-65.74), plagioclase index of alteration (PIA)(51.65-78.06), modified index of composition variation (ICVm)(0.67-1.24) and A-CN-K diagram reflect mild to moderate weathering of source rock. Discriminant function diagram, K-Rb bivariate diagram, A-CN-K diagram, Al2O3/TiO2 index(23.37-28.58), REE pattern similar to upper continental crust (UCC) and negative Eu anomaly deduce the felsic rocks as the major source for the dolomite-bearing mudstones. Under such geological constrain, it is concluded that the source materials are mostly derived from Yanshan fold and thrust belt.
-
Key words:
- Tanggu area /
- Shahejie Formation /
- dolomite-bearing mudstone /
- geochemistry /
- depositional setting /
- sedimentology
-
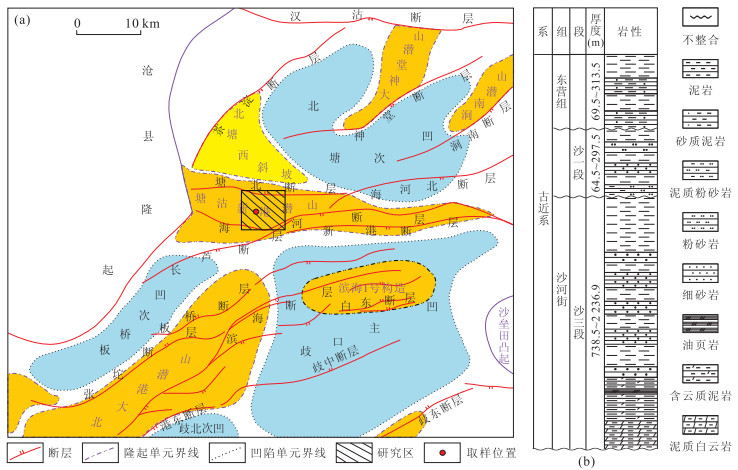
图 1 区域构造(a)和研究区古近系地层简图(b)
图a底图据周立宏等(2011)修改
Fig. 1. Geological sketch of study area (a) and generalized paleogene stratigraphic of the study area (b)
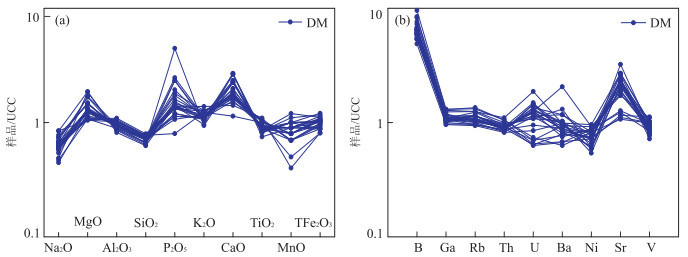
图 3 沙三段含云质泥岩主量元素氧化物(a)、微量元素(b)UCC标准化模式
UCC数据来自Rudnick and Gao(2003);DM.含云质泥岩(以下同)
Fig. 3. Distribution of UCC-nomalized major oxides (a) and trace elements (b) of Sha3 member dolomite-bearing mudstones
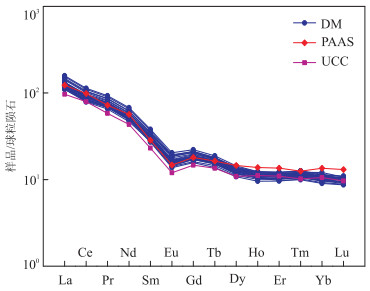
图 4 沙三段含云质泥岩稀土元素配分模式
球粒陨石、PAAS及UCC数据分别来自于Henderson(1984), Taylor and McLennan(1985)及Rudnick and Gao(2003)
Fig. 4. Chondrite-normalized REE pattern of Sha3 member dolomite-bearing mudstones
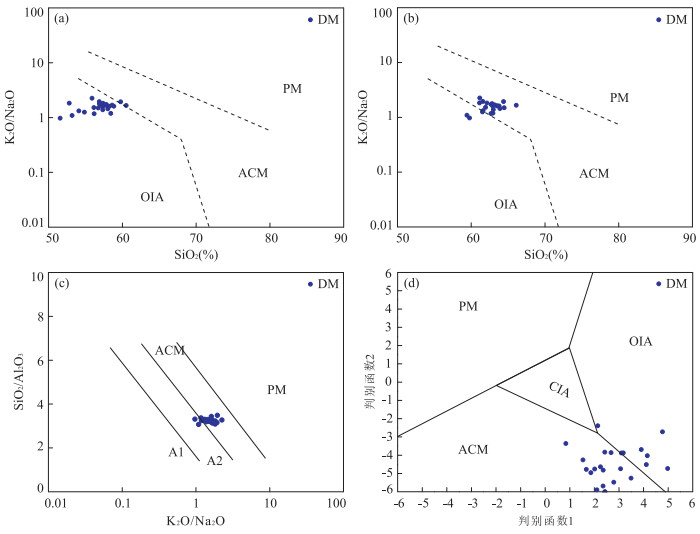
图 5 沙三段含云质泥岩构造背景判别
a.K2O/Na2O-SiO2双变量判别图,据Roser and Korsch(1986);b.K2O/Na2O-SiO2双变量图解,所有样品均经过白云石及LOI修正处理;c.SiO2/Al2O3-K2O/Na2O双变量判别图解,据Maynard et al.(1982),Roser and Korsch(1986);d.判别函数双变量图解,据Bhatia(1983);OIA.大洋岛弧边缘;ACM.主动大陆边缘;PM.被动大陆边缘;A1.原始岛弧;A2.进化岛弧;CIA.大陆岛弧;判别函数1=0.303-0.0447×SiO2%-0.972×TiO2%+0.008×Al2O3%-0.267×Fe2O3%+0.208×FeO%-3.082×MnO%+0.140×MgO%+0.195×CaO%+0.719×Na2O%-0.032×K2O%+7.510×P2O5%;判别函数2=43.57-0.421×SiO2%+1.988×TiO2%-0.526×Al2O3%-0.551×Fe2O3%-1.610×FeO%+2.720×MnO%+0.881×MgO-0.907×CaO%-0.117×Na2O%-1.840×K2O%+7.244×P2O5%
Fig. 5. Tectonic setting discrimination diagrams for Sha3 member dolomite-bearing mudstones
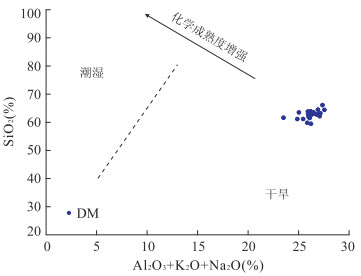
图 6 沙三段含云质泥岩古气候判别
Fig. 6. SiO2-(Al2O3+K2O+Na2O) bivariate paleoclimate discrimination for Sha3 member dolomite-bearing mudstones
图 7 沙三段含泥白云岩A-CN-K图解
Ga.辉长岩;T.英闪岩;Gr.花岗闪长岩;A.石英二长岩;G.花岗岩;箭头1.含云质泥岩风化趋势线;箭头2.理想风化趋势线;据Nesbitt and Young(1984)
Fig. 7. A-CN-K diagram for Sha3 member dolomite-bearing mudstones
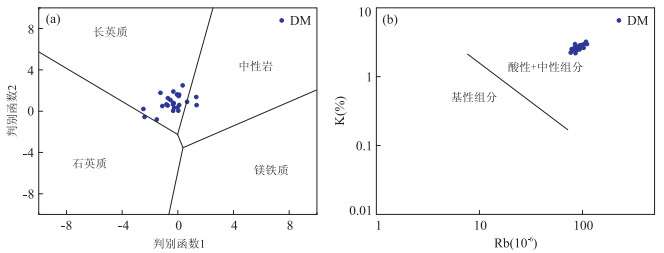
图 8 沙三段含云质泥岩物源属性判别图解
a.判别函数双变量图解,据Roser and Korsch(1988),判别函数1=-1.773×TiO2+0.607×Al2O3+0.760×TFe2O3-1.500×MgO+0.616×CaO+0.509×Na2O-1.224×K2O-9.090;判别函数2=0.445×TiO2+0.070×Al2O3-0.250×Fe2O3-1.142×MgO+0.438×CaO+1.475×Na2O+1.426×K2O-6.861;b.K-Rb双变量判别图解,据Floyd and Leveridge(1987)
Fig. 8. Provence discrimination diagrams for Sha3 member dolomite-bearing mudstones
表 1 沙三段含云质泥岩主量、微量和稀土元素分析结果
Table 1. Major element, trace element and rare-earth element (REE) analyses of Sha3 member dolomite-bearing mudstones
样品 Y1 Y2 Y3 Y4 Y5 Y6 Y7 Y8 Y9 Y10 Y11 Y12 Y13 Y14 Y15 Y16 Y17 Y18 Y19 Y20 Y21 Y22 Y23 Y24 Y25 Na2O 2.12 2.01 2.77 1.96 2.03 2.18 2.13 2.43 2.22 1.93 1.97 1.77 1.87 2.00 2.12 2.07 1.60 1.95 1.54 1.96 2.41 2.28 2.54 1.46 2.76 MgO 4.19 2.65 2.60 2.75 2.82 2.72 2.63 3.61 3.17 3.14 3.05 3.32 3.12 3.26 3.22 3.11 4.58 3.08 2.67 3.09 3.69 4.14 3.60 3.02 4.63 Al2O3 14.57 16.80 15.68 16.05 15.96 15.77 16.41 14.31 15.23 14.74 14.76 14.97 15.51 14.64 14.84 15.85 13.20 14.34 13.72 14.92 13.58 13.37 13.91 14.36 12.62 SiO2 47.54 52.99 50.82 49.50 50.29 51.60 51.43 43.90 48.65 48.03 48.99 47.92 48.82 46.87 48.86 51.60 42.57 49.29 47.83 48.13 44.82 44.08 46.84 46.98 41.76 P2O5 0.17 0.21 0.24 0.27 0.22 0.20 0.21 0.67 0.29 0.37 0.26 0.21 0.17 0.35 0.20 0.12 0.35 0.21 0.21 0.16 0.18 0.23 0.18 0.20 0.21 K2O 3.16 3.87 3.31 3.57 3.51 3.51 3.57 2.64 3.06 3.14 3.18 3.14 3.08 3.03 3.06 3.44 2.92 3.15 3.00 3.29 3.02 3.01 2.97 3.29 2.69 CaO 6.46 5.08 5.80 6.09 5.95 6.31 5.38 8.08 5.57 6.21 6.20 6.65 5.94 7.13 6.60 4.09 9.46 7.04 9.68 6.42 8.08 8.39 8.03 8.28 9.59 TiO2 0.57 0.70 0.67 0.65 0.65 0.66 0.69 0.52 0.60 0.57 0.58 0.57 0.60 0.57 0.58 0.64 0.53 0.59 0.48 0.57 0.54 0.53 0.57 0.55 0.54 MnO 0.07 0.04 0.07 0.08 0.08 0.07 0.07 0.10 0.09 0.08 0.08 0.09 0.09 0.09 0.09 0.05 0.10 0.09 0.09 0.08 0.10 0.10 0.10 0.12 0.11 Fe2O3 2.65 2.15 1.70 1.89 1.90 2.10 2.26 2.23 2.30 2.20 2.25 2.48 2.58 1.98 1.96 2.08 2.22 2.31 2.73 2.06 1.78 1.69 1.98 2.05 1.76 FeO 2.30 2.15 3.30 3.45 3.55 2.55 2.95 4.05 3.65 3.50 2.80 3.10 3.15 3.55 2.65 2.20 3.10 3.00 2.12 3.40 3.50 3.65 2.65 3.75 4.30 TFe2O3 5.21 4.54 5.37 5.72 5.85 4.93 5.54 6.73 6.36 6.09 5.36 5.93 6.08 5.93 4.90 4.52 5.67 5.64 5.09 5.84 5.67 5.75 4.92 6.22 6.54 H2O+ 3.66 4.88 5.14 5.28 5.04 4.74 5.20 6.02 6.44 7.68 7.76 6.24 5.64 6.22 5.80 8.66 5.70 4.98 5.38 4.80 6.04 7.22 5.56 5.42 5.16 H2O- 2.09 3.38 2.79 2.79 3.20 3.25 3.20 2.64 2.83 2.35 2.55 2.90 3.01 2.73 2.48 2.44 2.52 3.23 3.55 2.77 1.90 1.77 1.91 3.02 1.71 LOI 15.59 10.90 12.47 13.11 12.35 11.84 11.65 16.84 14.54 15.44 15.32 15.18 14.47 15.88 15.28 14.18 18.73 14.37 15.42 15.31 17.64 17.86 16.05 15.26 18.40 SUM 99.39 99.55 99.43 99.37 99.31 99.51 99.38 99.38 99.37 99.35 99.44 99.40 99.40 99.35 99.46 99.43 99.36 99.42 99.49 99.39 99.34 99.33 99.42 99.32 99.37 ICV 1.46 1.08 1.27 1.26 1.27 1.25 1.18 1.65 1.34 1.40 1.34 1.40 1.30 1.46 1.35 1.09 1.84 1.46 1.61 1.39 1.69 1.77 1.59 1.56 2.09 ICVm 0.80 0.74 0.91 0.88 0.88 0.87 0.83 1.07 0.88 0.92 0.88 0.90 0.85 0.96 0.86 0.67 1.04 0.98 1.17 0.92 1.07 1.06 1.01 1.08 1.24 K2O/Al2O3 0.22 0.23 0.21 0.22 0.22 0.22 0.22 0.18 0.20 0.21 0.22 0.21 0.20 0.21 0.21 0.22 0.22 0.22 0.22 0.22 0.22 0.23 0.21 0.23 0.21 CIA 65.74 63.88 57.67 60.86 60.85 58.95 62.21 57.06 64.62 62.09 60.63 61.86 63.61 59.73 59.41 71.33 61.02 59.32 62.24 59.83 54.79 55.39 54.57 63.17 51.26 PIA 42.21 50.08 44.43 45.96 46.15 44.23 48.52 37.95 46.33 43.89 43.76 43.49 46.69 41.09 42.62 52.75 33.56 40.52 34.17 43.21 35.55 34.68 36.24 38.16 30.59 Al2O3/TiO2 25.56 24.00 23.40 24.69 24.55 23.89 23.78 27.52 25.38 25.86 25.45 26.26 25.85 25.68 25.59 24.77 24.91 24.31 28.58 26.18 25.15 25.23 24.40 26.11 23.37 B 123.95 162.92 113.11 116.06 111.03 103.48 140.49 96.50 91.55 100.73 101.27 100.89 110.55 106.79 100.44 129.64 109.83 103.11 106.08 143.62 92.51 111.17 90.75 109.36 83.14 Ga 23.05 23.57 21.47 21.07 22.35 19.67 23.48 20.33 19.03 19.02 18.43 19.73 19.23 17.97 19.61 20.45 17.40 18.88 18.14 19.20 18.80 19.06 18.37 20.03 17.35 Rb 102.70 108.00 85.56 84.70 102.20 100.70 110.70 85.61 81.66 88.28 88.23 88.12 85.14 79.04 82.99 94.40 79.71 93.94 93.62 94.95 92.59 78.89 88.94 92.42 76.85 Th 11.53 11.00 9.30 9.56 10.52 9.96 11.03 9.88 8.72 9.28 9.05 9.48 9.33 8.92 9.60 9.57 8.98 9.16 9.30 9.40 10.00 9.05 9.99 10.27 8.60 U 5.06 1.90 1.73 1.89 1.76 1.71 1.99 3.08 3.05 3.66 3.59 3.33 3.29 3.89 4.03 3.37 3.93 2.57 3.43 3.39 3.08 3.45 2.93 2.92 2.30 Ba 616.51 484.65 494.22 592.81 429.70 417.92 397.60 734.55 508.70 736.46 569.91 554.52 467.90 532.80 520.11 1293.75 598.20 559.70 622.74 545.75 619.90 640.37 821.17 646.84 618.28 Ni 38.77 40.23 34.89 43.09 33.93 40.73 38.69 39.19 39.66 35.95 38.62 34.53 41.38 34.12 28.95 43.42 31.24 33.15 45.34 38.57 27.53 34.16 28.37 25.40 27.81 Sr 756.56 406.28 355.58 551.98 370.04 392.43 343.20 857.87 605.17 732.26 704.79 744.21 659.29 729.04 742.62 569.19 1032.75 725.53 863.10 612.90 763.58 834.26 776.89 687.98 670.93 V 93.68 94.68 96.81 106.69 108.49 81.50 95.76 97.76 81.44 83.47 77.72 87.75 86.88 82.21 75.86 85.27 86.95 99.66 96.24 85.60 75.12 79.91 69.58 89.58 92.18 相当B 267.85 313.21 237.47 233.06 225.13 209.82 282.12 235.44 201.97 218.56 217.97 218.90 242.85 237.10 221.58 265.97 249.91 223.27 237.07 302.65 205.84 247.90 204.17 230.45 200.17 Sr/Ba 1.23 0.84 0.72 0.93 0.86 0.94 0.86 1.17 1.19 0.99 1.24 1.34 1.41 1.37 1.43 0.44 1.73 1.30 1.39 1.12 1.23 1.30 0.95 1.06 1.09 Fepy-apx 2.29 - 0.74 0.88 0.44 0.38 0.16 1.84 0.78 0.86 0.72 1.04 1.04 1.46 1.51 - 2.39 1.21 1.55 1.90 2.47 1.89 2.02 1.53 2.82 DOPapx 0.38 - 0.11 0.12 0.06 0.07 0.02 0.19 0.09 0.11 0.11 0.13 0.13 0.19 0.27 - 0.33 0.17 0.27 0.25 0.35 0.26 0.35 0.18 0.33 自生U 1.22 - - - - - - - 0.14 0.56 0.58 0.17 0.18 0.92 0.83 0.18 0.93 - 0.33 0.25 - 0.43 - - - U/Th 0.44 0.17 0.19 0.20 0.17 0.17 0.18 0.31 0.35 0.39 0.40 0.35 0.35 0.44 0.42 0.35 0.44 0.28 0.37 0.36 0.31 0.38 0.29 0.28 0.27 Ni/V 0.41 0.42 0.36 0.40 0.31 0.50 0.40 0.40 0.49 0.43 0.50 0.39 0.48 0.42 0.38 0.51 0.36 0.33 0.47 0.45 0.37 0.43 0.41 0.28 0.30 La 39.45 44.76 49.14 45.83 43.76 41.14 47.86 35.45 40.08 37.14 36.48 36.16 40.53 38.48 38.52 34.15 37.50 40.33 34.14 38.65 36.93 37.84 34.44 41.09 38.83 Ce 72.54 83.40 91.61 85.16 81.56 76.80 89.18 65.96 73.63 67.46 67.37 65.91 75.33 71.53 71.61 65.67 67.70 74.37 63.53 71.49 67.87 69.89 65.04 77.74 71.45 Pr 9.14 10.51 11.34 10.48 10.01 9.48 11.01 8.44 9.40 8.72 8.56 8.38 9.32 8.93 9.01 8.33 8.61 9.49 7.96 9.05 8.66 8.84 8.31 9.86 9.22 Nd 32.13 37.09 40.52 37.13 35.5 33.34 39.31 30.27 33.15 30.69 30.25 29.53 33.15 31.58 31.89 29.09 30.71 33.42 28.01 31.94 30.81 31.69 29.70 34.70 32.75 Sm 5.90 6.83 7.42 6.83 6.54 6.06 7.21 5.76 6.23 5.83 5.75 5.56 6.36 5.97 6.06 5.27 6.01 6.23 5.40 5.96 5.81 5.95 5.61 6.65 6.00 Eu 1.15 1.38 1.50 1.39 1.36 1.23 1.43 1.12 1.20 1.13 1.13 1.05 1.25 1.18 1.18 1.01 1.14 1.24 1.04 1.16 1.11 1.16 1.08 1.31 1.23 Gd 4.61 5.30 5.74 5.50 5.35 4.60 5.43 4.71 4.86 4.74 4.48 4.42 5.02 4.82 4.8 4.06 4.85 4.88 4.17 4.76 4.55 4.75 4.41 5.10 4.44 Tb 0.76 0.83 0.89 0.85 0.82 0.74 0.85 0.75 0.78 0.75 0.73 0.72 0.83 0.77 0.79 0.65 0.82 0.78 0.69 0.78 0.75 0.77 0.72 0.84 0.75 Dy 4.04 4.26 4.64 4.43 4.26 3.77 4.33 4.02 4.00 3.95 3.86 3.72 4.35 4.14 4.16 3.48 4.29 4.00 3.59 4.21 4.03 4.08 3.81 4.47 3.84 Ho 0.77 0.79 0.89 0.87 0.83 0.74 0.86 0.79 0.80 0.78 0.75 0.75 0.86 0.82 0.81 0.69 0.84 0.79 0.72 0.83 0.79 0.81 0.75 0.89 0.76 Er 2.27 2.25 2.55 2.43 2.39 2.06 2.45 2.36 2.29 2.27 2.16 2.12 2.53 2.36 2.40 2.02 2.39 2.23 2.12 2.43 2.25 2.33 2.14 2.54 2.16 Tm 0.36 0.36 0.39 0.39 0.38 0.32 0.39 0.38 0.38 0.37 0.34 0.35 0.41 0.37 0.38 0.33 0.39 0.35 0.34 0.38 0.36 0.37 0.35 0.41 0.35 Yb 2.10 2.10 2.36 2.26 2.20 1.89 2.27 2.26 2.22 2.18 2.08 2.11 2.41 2.25 2.27 1.94 2.53 2.13 2.02 2.27 2.13 2.19 2.05 2.34 2.01 Lu 0.33 0.31 0.34 0.35 0.33 0.29 0.34 0.35 0.34 0.34 0.31 0.31 0.36 0.34 0.34 0.29 0.35 0.31 0.30 0.35 0.33 0.34 0.31 0.37 0.31 Y 21.24 21.74 23.98 22.58 21.90 19.95 22.20 21.62 21.56 20.90 20.11 19.74 23.47 22.25 21.57 17.68 21.22 21.16 19.12 22.38 20.71 21.78 19.99 24.51 20.14 ∑REE 175.5 200.2 219.3 203.9 195.3 182.5 212.9 162.6 179.4 166.3 164.3 161.1 182.7 173.5 174.2 157 168.1 180.5 154 174.3 166.4 171 158.7 188.3 174.1 LaN/YbN 12.65 14.38 14.06 13.64 13.43 14.71 14.23 10.59 12.19 11.50 11.83 11.53 11.33 11.51 11.43 11.90 10.01 12.78 11.42 11.47 11.67 11.63 11.34 11.84 13.03 L/H 10.52 11.35 11.32 10.94 10.79 11.66 11.58 9.42 10.45 9.82 10.16 10.11 9.90 9.93 9.92 10.67 9.21 10.68 10.04 9.88 9.95 9.93 9.91 10.1 10.91 δEu 0.67 0.70 0.70 0.69 0.70 0.71 0.70 0.66 0.66 0.66 0.68 0.65 0.68 0.67 0.67 0.67 0.64 0.69 0.67 0.66 0.66 0.67 0.67 0.69 0.73 注:主量元素单位为%;微量元素单位为10-6;稀土元素单位为10-6. 表 2 沙三段含云质泥岩主量元素氧化物与微量元素皮尔逊积矩相关系数
Table 2. Pearson correlation coefficient of major and trace element in Sha3 member dolomite-bearing mudstones
Na2O MgO Al2O3 SiO2 P2O5 K2O CaO TiO2 MnO Fe2O3 FeO H2O+ H2O- LOI B Ga Rb Th U Ba Ni Sr V Na2O 1.00 MgO 0.23 1.00 Al2O3 -0.09 -0.78 1.00 SiO2 -0.14 -0.86 0.91 1.00 P2O5 0.09 0.16 -0.16 -0.38 1.00 K2O -0.23 -0.69 0.85 0.85 -0.43 1.00 CaO -0.04 0.60 -0.89 -0.84 0.23 -0.71 1.00 TiO2 0.18 -0.60 0.89 0.82 -0.28 0.84 -0.82 1.00 MnO -0.03 0.50 -0.77 -0.80 0.24 -0.75 0.79 -0.74 1.00 Fe2O3 -0.56 -0.12 0.09 0.16 0.02 -0.05 -0.06 -0.18 -0.14 1.00 FeO 0.28 0.32 -0.35 -0.58 0.46 -0.45 0.33 -0.27 0.64 -0.47 1.00 H2O+ -0.05 0.03 -0.08 -0.07 0.17 -0.17 -0.16 -0.16 -0.01 -0.13 0.07 1.00 H2O- -0.54 -0.77 0.61 0.64 -0.01 0.54 -0.32 0.41 -0.35 0.44 -0.31 -0.29 1.00 LOI 0.06 0.85 -0.93 -0.94 0.26 -0.87 0.76 -0.88 0.72 -0.09 0.41 0.26 -0.71 1.00 B -0.26 -0.38 0.62 0.56 -0.27 0.74 -0.52 0.55 -0.72 0.11 -0.44 -0.21 0.40 -0.56 1.00 Ga 0.06 -0.43 0.74 0.62 -0.12 0.71 -0.62 0.70 -0.64 0.06 -0.29 -0.36 0.34 -0.72 0.68 1.00 Rb -0.17 -0.49 0.58 0.65 -0.34 0.71 -0.45 0.54 -0.61 0.29 -0.54 -0.38 0.49 -0.67 0.64 0.74 1.00 Th -0.05 -0.21 0.45 0.40 -0.18 0.53 -0.31 0.41 -0.41 0.18 -0.40 -0.47 0.20 -0.47 0.54 0.83 0.83 1.00 U -0.30 0.50 -0.46 -0.41 0.07 -0.50 0.22 -0.63 0.21 0.41 -0.16 0.25 -0.40 0.61 -0.14 -0.33 -0.30 -0.11 1.00 Ba 0.05 0.22 -0.19 -0.13 0.00 -0.18 -0.03 -0.22 -0.07 -0.06 -0.16 0.60 -0.41 0.31 -0.06 -0.18 -0.15 -0.11 0.30 1.00 Ni -0.21 -0.47 0.54 0.52 0.06 0.38 -0.48 0.31 -0.64 0.50 -0.43 0.08 0.48 -0.48 0.44 0.31 0.30 0.08 -0.07 0.06 1.00 Sr -0.26 0.67 -0.81 -0.77 0.33 -0.78 0.70 -0.90 0.62 0.25 0.11 0.24 -0.43 0.87 -0.46 -0.66 -0.56 -0.38 0.74 0.31 -0.27 1.00 V -0.14 -0.28 0.34 0.22 0.16 0.33 -0.11 0.30 -0.21 0.13 0.11 -0.43 0.51 -0.41 0.32 0.52 0.31 0.27 -0.45 -0.22 0.33 -0.36 1.00 -
Adams, J.A.S., Weaver, C.E., 1958. Thorium-to-Uranium Ratios as Indicators of Sedimentary Processes, Example of Concept of Geochemical Facies. AAPG Bulletin, 42(2): 387-430. doi: 10.1306/0bda5a89-16bd-11d7-8645000102c1865d Algeo, T.J., Maynard, J.B., 2004. Trace-Element Behavior and Redox Facies in Core Shales of Upper Pennsylvanian Kansas-Type Cyclothems. Chemical Geology, 206(3-4): 289-318. doi: 10.1016/j.chemgeo.2003.12.009 Bai, Y.F., Wang, H., Wang, Z.S., et al., 2011. Thermal Evolution Modeling and Characteristic of Source Rock of Paleogene in Beitang Sag. Earth Science—Journal of China University of Geosciences, 36(3): 565-571 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-DQKX201103013.htm Berner, R.A., 1970. Sedimentary Pyrite Formation. American Journal of Science, 268(1): 1-23. doi: 10.2475/ajs.268.1.1 Bhatia, M.R., 1983. Plate Tectonics and Geochemical Composition of Sandstones. The Journal of Geology, 91(6): 611-627. doi: 10.1086/628815 Bhatia, M.R., 1985. Rare Earth Element Geochemistry of Australian Paleozoic Graywackes and Mudrocks: Provenance and Tectonic Control. Sedimentary Geology, 45(1-2): 97-113. doi: 10.1016/0037-0738(85)90025-9 Couch, E.L., 1971. Calculation of Paleosalinities from Boron and Clay Mineral Data. AAPG Bulletin, 55(10): 1829-1837. doi: 10.1306/819a3dac-16c5-11d7-8645000102c1865d Cox, R., Lowe, D.R., Cullers, R.L., 1995. The Influence of Sediment Recycling and Basement Composition on Evolution of Mudrock Chemistry in the Southwestern United States. Geochimica et Cosmochimica Acta, 59(14): 2919-2940. doi: 10.1016/0016-7037(95)00185-9 Cullers, R.L., 2000. The Geochemistry of Shales, Siltstones and Sandstones of Pennsylvanian-Permian Age, Colorado, USA: Implications for Provenance and Metamorphic Studies. Lithos, 51(3): 181-203. doi: 10.1016/S0024-4937(99)00063-8 Curtis, C.D., 1964. Studies on the Use of Boron as a Paleoenvironmental Indicator. Geochimica et Cosmochimica Acta, 28(7): 1125-1137. doi: 10.1016/0016-7037(64)90064-x Deng, H.W., Qian, K., 1993. Sedimentary Geochemistry and Environment Analysis. Gansu Science and Technology Press, Lanzhou, 18-31 (in Chinese). Deng, R.J., Xu, B., Qi, J.F., et al., 2006. Sedimentation Characteristics and Factors Affecting the Reservoir in Palaeogene Shasan Member of Beitang Sag, Huanghua Depression. Acta Petrologica et Mineralogica, 25(3): 230-236 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-YSKW200603007.htm Deng, R.J., Xu, B., Yang, Y., et al., 2005. Paleogene Source Rock Characteristic and Its Evolution in Beitang Sag, Huanghua Depression. Petroleum Geology and Recovery Efficiency, 12(4): 35-38 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-YQCS200504012.htm Fedo, C.M., Nesbitt, H.W., Young, G.M., 1995. Unraveling the Effects of Potassium Metasomatism in Sedimentary Rocks and Paleosols, with Implications for Paleoweathering Conditions and Provenance. Geology, 23(10): 921-924. doi:10.1130/0091-7613(1995)023<0921:Uteopm>2.3.co;2 Floyd, P.A., Leveridge, B.E., 1987. Tectonic Environment of the Devonian Gramscatho Basin, South Cornwall: Framework Mode and Geochemical Evidence from Turbiditic Sandstones. Journal of the Geological Society, 144(4): 531-542. doi: 10.1144/gsjgs.144.4.0531 Frederickson, A.F., Reynolds, R.C., 1960. Geochemical Method for Determining Paleosalinity. Clays and Clay Minerals, 9: 203-213. doi: 10.1016/b978-0-08-009351-2.50023-4 Ghosh, S., Sarkar, S., 2010. Geochemistry of Permo-Triassic Mudstone of the Satpura Gondwana Basin, Central India: Clues for Provenance. Chemical Geology, 277(1-2): 78-100. doi: 10.1016/j.chemgeo.2010.07.012 Guo, H., Li, M., Li, S.L., et al., 2002. Study on Characteristics of Intra-Plate Orogen: Taking Yanshan and Dabieshan Orogen Belt for Example. Geological Publishing House, Beijing, 48-62 (in Chinese). Harder, H., 1970. Boron Content of Sediments as a Tool in Facies Analysis. Sedimentary Geology, 4(1-2): 153-175. doi: 10.1016/0037-0738(70)90009-6 Harnois, L., 1988. The CIW Index: A New Chemical Index of Weathering. Sedimentary Geology, 55(3-4): 319-322. doi: 10.1016/0037-0738(88)90137-6 Hayashi, K.I., Fujisawa, H., Holland, H.D., et al., 1997. Geochemistry of 1.9 Ga Sedimentary Rocks from Northeastern Labrador, Canada. Geochimica et Cosmochimica Acta, 61(19): 4115-4137. doi: 10.1016/S0016-7037(97)00214-7 Henderson, P., 1984. Rare Earth Element Geochemistry. Elsevier, Amsterdam, 52-71. Hofer, G., Wagreich, M., Neuhuber, S., 2013. Geochemistry of Fine-Grained Sediments of the Upper Cretaceous to Paleogene Gosau Group (Austria, Slovakia): Implications for Paleoenvironmental and Provenance Studies. Geoscience Frontiers, 4(4): 449-468. doi: 10.1016/j.gsf.2012.11.009 Hu, S.X., 1992. Ponderation on Problems about Evolution of Mesozoic-Cenozoic Tectono-Magmatism of Active Continetal Margin in East China. Earth Science—Journal of China University of Geosciences, 17(Suppl): 40-46 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-DQKX1992S1006.htm Huang, C.Y., Wang, H., Gao, J.R., et al., 2008. Tectonic Evolution and Its Controlling over Sequence Filling Pattern of Paleogene in Beitang Sag. Journal of China University of Petroleum, 32(3): 7-13 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-SYDX200803003.htm Huang, C.Y., Wang, H., Zhou, L.H., et al., 2009. Provenance System Characters of the Third Member of Shahejie Formation in the Paleogene in Beitang Sag. Earth Science—Journal of China University of Geosciences, 34(6): 975-984 (in Chinese with English abstract). doi: 10.3799/dqkx.2009.111 Imchen, W., Thong, G.T., Pongen, T., 2014. Provenance, Tectonic Setting and Age of the Sediments of the Upper Disang Formation in the Phek District, Nagaland. Journal of Asian Earth Sciences, 88: 11-27. doi: 10.1016/j.jseaes.2014.02.027 Jian, X., Guan, P., Zhang, W., et al., 2013. Geochemistry of Mesozoic and Cenozoic Sediments in the Northern Qaidam Basin, Northeastern Tibetan Plateau: Implications for Provenance and Weathering. Chemical Geology, 360-361: 74-88. doi: 10.1016/j.chemgeo.2013.10.011 Jones, B., Manning, D.A.C., 1994. Comparison of Geochemical Indices Used for the Interpretation of Palaeoredox Conditions in Ancient Mudstones. Chemical Geology, 111(1-4): 111-129. doi: 10.1016/0009-2541(94)90085-X Li, D.W., 2006. Neotectonism and Hydrocarbon Accumulation in Huanghua Depression, China. China University of Geosciences Press, Wuhan, 23-30 (in Chinese). Lerman, A., 1966. Boron in Clays and Estimation of Paleosalinities. Sedimentology, 6(4): 267-286. doi: 10.1111/j.1365-3091.1966.tb01895.x Ma, J., Gao, Y., Deng, R.J., et al., 1999. Reservoir Evaluation and Favorable Belt Prediction for Es3 Formation of Beitang Sag. Petroleum Exploration and Development, 26(6): 36-38 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-SKYK199906012.htm Maynard, J.B., Valloni, R., Yu, H.S., 1982. Composition of Modern Deep-Sea Sands from Arc-Related Basins. Geological Society, London, Special Publications, 10(1): 551-561. doi: 10.1144/gsl.sp.1982.010.01.36 McLennan, S.M., Hemming, S., McDaniel, D.K., et al., 1993. Geochemical Approaches to Sedimentation, Provenance, and Tectonics. Geological Society of America Special Papers, 284: 21-40.10.1130/SPE284-p21 doi: 10.1130/SPE284-p21 Moosavirad, S.M., Janardhana, M.R., Sethumadhav, M.S., et al., 2011. Geochemistry of Lower Jurassic Shales of the Shemshak Formation, Kerman Province, Central Iran: Provenance, Source Weathering and Tectonic Setting. Chemie der Erde-Geochemistry, 71(3): 279-288. doi: 10.1016/j.chemer.2010.10.001 Nesbitt, H.W., Young, G.M., 1982. Early Proterozoic Climates and Plate Motions Inferred from Major Element Chemistry of Lutites. Nature, 299(5885): 715-717. doi: 10.1038/299715a0 Nesbitt, H.W., Young, G.M., 1984. Prediction of Some Weathering Trends of Plutonic and Volcanic Rocks Based on Thermodynamic and Kinetic Considerations. Geochimica et Cosmochimica Acta, 48(7): 1523-1534. doi: 10.1016/0016-7037(84)90408-3 Nesbitt, H.W., Young, G.M., 1989. Formation and Diagenesis of Weathering Profiles. The Journal of Geology, 97(2): 129-147. doi: 10.1086/629290 Peng, L.C., Han, D.X., Pu, R.L., et al., 1999. w(Sr)/w(Ba) Value of Continental Brackish Lake Deposit and Its Geological Significance. Journal of China University of Mining & Technology, 28(1): 50-52 (in Chinese with English abstract). http://www.cqvip.com/Main/Detail.aspx?id=3430741 Roser, B.P., Korsch, R.J., 1986. Determination of Tectonic Setting of Sandstone-Mudstone Suites Using SiO2 Content and K2O/Na2O Ratio. The Journal of Geology, 94(5): 635-650. doi: 10.1086/629071 Roser, B.P., Korsch, R.J., 1988. Provenance Signatures of Sandstone-Mudstone Suites Determined Using Discriminant Function Analysis of Major-Element Data. Chemical Geology, 67(1-2): 119-139. doi: 10.1016/0009-2541(88)90010-1 Rudnick, R.L., Gao, S., 2003. Composition of the Continental Crust. In: Holland, H.D., Turekian, K.K., eds., Treatise on Geochemistry. Pergamon, Oxford, 1-64 Sharma, A., Sensarma, S., Kumar, K., et al., 2013. Mineralogy and Geochemistry of the Mahi River Sediments in Tectonically Active Western India: Implications for Deccan Large Igneous Province Source, Weathering and Mobility of Elements in a Semi-Arid Climate. Geochimica et Cosmochimica Acta, 104: 63-83. doi: 10.1016/j.gca.2012.11.004 Siever, R., 1979. Plate-Tectonic Controls on Diagenesis. The Journal of Geology, 87(2): 127-155. doi: 10.1086/628405 Song, W.J., Dai, S.F., Zhao, L., et al., 2014. Determination of Boron in Coal Samples with Microwave Digestion by Inductively Coupled Plasma-Mass Spectrometry. Rock and Mineral Analysis, 33(3): 327-331 (in Chinese with English abstract). doi: 10.1021/ef500912a Song, X.M., Qian, X.L., 1995. Tectonic Origin and Evolution of Meso-Cenozoic Bohai Bay Basin and Its Adjacent Area. Earth Science Frontiers, 2(1-2): 194 (in Chinese). Suttner, L.J., Dutta, P.K., 1986. Alluvial Sandstone Composition and Paleoclimate, I. Framework Mineralogy. Journal of Sedimentary Research, 56(3): 329-345. doi: 10.1306/212f8909-2b24-11d7-8648000102c1865d Taylor, S.R., McLennan, S.M., 1985. The Continental Crust: Its Composition and Evolution. Blackwell, London, 57-72. Tribovillard, N., Algeo, T.J., Lyons, T., et al., 2006. Trace Metals as Paleoredox and Paleoproductivity Proxies: An Update. Chemical Geology, 232(1-2): 12-32. doi: 10.1016/j.chemgeo.2006.02.012 van de Kamp, P.C.V.D., Leake, B.E., 1985. Petrography and Geochemistry of Feldspathic and Mafic Sediments of the Northeastern Pacific Margin. Transactions of the Royal Society of Edinburgh: Earth Sciences, 76(4): 411-449. doi: 10.1017/s0263593300010646 Walker, C.T., 1968. Evaluation of Boron as a Paleosalinity Indicator and Its Application to Offshore Prospects. AAPG Bulletin, 52(5): 751-766. doi: 10.1306/5d25c45d-16c1-11d7-8645000102c1865d Walker, C.T., Price, N.B., 1963. Departure Curves for Computing Paleosalinity from Boron in Illites and Shale. AAPG Bulletin, 47(5): 833-841. doi: 10.1306/bc743a93-16be-11d7-8645000102c1865d Wang, S.J., Huang, X.Z., Tuo, J.C., et al., 1997. Evolutional Characteristics and Their Paleoclimate Significance of Trace Elements in the Hetaoyuan Formation, Biyang Depression. Acta Sedimentologica Sinica, 15(1): 65-70 (in Chinese with English abstract). http://d.wanfangdata.com.cn/periodical/QK199700005821 Wignall, P.B., Myers, K.J., 1988. Interpreting Benthic Oxygen Levels in Mudrocks: A New Approach. Geology, 16(5): 452-455. doi:10.1130/0091-7613(1988)016<0452:ibolim>2.3.co;2 Wignall, P.B., Twitchett, R.J., 1996. Oceanic Anoxia and the End Permian Mass Extinction. Science, 272(5265): 1155-1158. doi: 10.1126/science.272.5265.1155 Xiao, K.Y., Deng, R.Y., Yang, H., et al., 2004. Petroleum Geological Role of Magmatic Activities in Xin'gang Prospect Area, Beitang Sag. Petroleum Exploration and Development, 31(2): 25-28 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-SKYK200402006.htm Xu, Z.J., Cheng, R.H., Wang, L.L., et al., 2010. Elemental Geochemical Characteristics of Tuffaceous Sediments and Tectonic Setting of Tangxia Formation of Middle Jurassic in Dongguan, Guangdong Province. Acta Petrologica Sinica, 26(1): 352-360 (in Chinese with English abstract). Yan, Y., Xia, B., Lin, G., et al., 2007. Geochemistry of the Sedimentary Rocks from the Nanxiong Basin, South China and Implications for Provenance, Paleoenvironment and Paleoclimate at the K/T Boundary. Sedimentary Geology, 197(1-2): 127-140. doi: 10.1016/j.sedgeo.2006.09.004 Yang, X.F., He, D.F., Wang, Q.C., et al., 2012. Provenance and Tectonic Setting of the Carboniferous Sedimentary Rocks of the East Junggar Basin, China: Evidence from Geochemistry and U-Pb Zircon Geochronology. Gondwana Research, 22(2): 567-584. doi: 10.1016/j.gr.2011.11.001 Zhai, G.M., Ning, J.G., Jin, J.Q., et al., 2002. Tectonic Evolution of Plate and Formation and Evaluation of Petroliferous Basins. Petroleum Industry Press, Beijing, 33-35 (in Chinese). Zhang, T.T., Wang, H., Yue, Y., et al., 2009. Cenozoic Subsidence Features of Beitang Sag and Relationship with Tectonic Evolution. Journal of Earth Science, 20(4): 746-754. doi: 10.1007/s12583-009-0061-9 Zhou, L.H., Lu, Y., Xiao, D.Q., et al., 2011. Basinal Texture Structure of Qikou Sag in Bohai Bay Basin and Its Evolution. Natural Gas Geoscience, 22(3): 373-382 (in Chinese with English abstract). http://www.researchgate.net/publication/285151279_Basinal_texture_structure_of_Qikou_Sag_in_Bohai_Bay_Basin_and_its_evolution 白云风, 王华, 王振升, 等, 2011. 北塘凹陷古近系烃源岩特征及热演化史模拟. 地球科学——中国地质大学学报, 36(3): 565-571. https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX201103013.htm 邓宏文, 钱凯, 1993. 沉积地球化学与环境分析. 兰州: 甘肃科学技术出版社, 18-23. 邓荣敬, 徐备, 漆家福, 等, 2006. 北塘凹陷古近系沙河街组三段沉积特征及储层的影响因素. 岩石矿物学杂志, 25(3): 230-236. doi: 10.3969/j.issn.1000-6524.2006.03.008 邓荣敬, 徐备, 杨桦, 等, 2005. 黄骅坳陷北塘凹陷古近系烃源岩特征与演化. 油气地质与采收率, 12(4): 35-38. doi: 10.3969/j.issn.1009-9603.2005.04.012 郭华, 李明, 李守林, 等, 2002. 板内造山带主要构造特征研究: 以燕山和大别山造山带为例. 北京: 地质出版社, 48-62. 胡受奚, 1992. 有关中国东部中、新生代活动大陆边缘构造—岩浆作用演化问题沉思录. 地球科学——中国地质大学学报, 17(增刊): 40-46. https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX1992S1006.htm 黄传炎, 王华, 高嘉瑞, 等, 2008. 北塘凹陷古近系构造演化及其对层序充填样式的控制. 中国石油大学学报(自然科学版), 32(3): 7-13. doi: 10.3321/j.issn:1673-5005.2008.03.002 黄传炎, 王华, 周立宏, 等, 2009. 北塘凹陷古近系沙河街组三段物源体系分析. 地球科学——中国地质大学学报, 34(6): 975-984. 李大伟, 2006. 新构造运动与黄骅坳陷油气成藏. 武汉: 中国地质大学出版社, 23-30. 马杰, 高勇, 邓荣敬, 等, 1999. 北塘凹陷沙三段储集层评价及有利相带预测. 石油勘探与开发, 26(6): 36-38. doi: 10.3321/j.issn:1000-0747.1999.06.010 彭立才, 韩德馨, 濮人龙, 等, 1999. 陆相咸化湖泊沉积中锶/钡比值及其地质意义. 中国矿业大学学报, 28(1): 50-52. doi: 10.3321/j.issn:1000-1964.1999.01.012 宋伟娇, 代世峰, 赵蕾, 等, 2014. 微波消解-电感耦合等离子体质谱法测定煤中的硼. 岩矿测试, 33(3): 327-331. doi: 10.3969/j.issn.0254-5357.2014.03.007 宋新民, 钱祥麟, 1995. 中新生代渤海湾盆地及其邻区构造成因和演化. 地学前缘, 2(1-2): 194. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY502.013.htm 王随继, 黄杏珍, 妥进才, 等, 1997. 泌阳凹陷核桃园组微量元素演化特征及其古气候意义. 沉积学报, 15(1): 65-70. https://www.cnki.com.cn/Article/CJFDTOTAL-CJXB701.011.htm 肖坤叶, 邓荣敬, 杨烨, 等, 2004. 北塘凹陷新港探区新生代岩浆活动的石油地质意义. 石油勘探与开发, 31(2): 25-28. doi: 10.3321/j.issn:1000-0747.2004.02.006 许中杰, 程日辉, 王嘹亮, 等, 2010. 广东东莞地区中侏罗统塘厦组凝灰质沉积物的元素地球化学特征及构造背景. 岩石学报, 26(1): 352-360. https://www.cnki.com.cn/Article/CJFDTOTAL-YSXB201001040.htm 翟光明, 宁建国, 靳久强, 等, 2002. 板块构造演化与含油气盆地形成和评价. 北京: 石油工业出版社, 33-35. 周立宏, 卢异, 肖敦清, 等, 2011. 渤海湾盆地歧口凹陷盆地结构构造及演化. 天然气地球科学, 22(3): 373-382. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX201103002.htm -








 下载:
下载: