Effect of Phenolic Acids Derived from Peatland on Surface Behavior of Iron and Its Significance:A Case Study in Hani Peatland
-
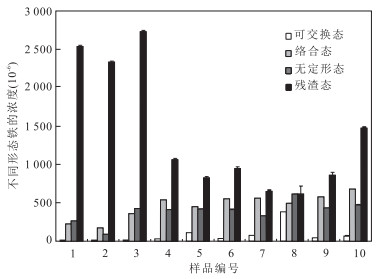
摘要: 大量研究表明,溶解性有机质与铁的螯合对生物可利用性铁的输出有重要影响.然而,对于天然有机质,尤其是泥炭沼泽源的酚类物质,与铁相互作用的地球化学机制仍然缺乏研究.以长白山西麓哈尼泥炭沼泽为研究对象,调查了泥炭沼泽源水体可溶性总铁、亚铁、水溶性总酚等理化指标.同时,测定了泥炭中酚酸的组成及含量,分析对比泥炭与土壤中铁的主要赋存形态.并开展了酚铁相互作用模拟实验,研究了泥炭沼泽源水体中酚铁相互作用机制.结果表明:哈尼泥炭沼泽水体中亚铁浓度与水溶性总酚浓度显著相关,说明水溶性总酚对亚铁的存在及运移有重要影响.哈尼泥炭中含有原儿茶酸、咖啡酸、没食子酸、龙胆酸、丁香酸、阿魏酸、对羟基苯甲酸、对香豆酸、水杨酸、香草酸等多种酚酸.其中,具有儿茶酚或没食子酰基结构的原儿茶酸、咖啡酸和没食子酸能与亚铁形成稳定螯合物,是泥炭沼泽源水体中Fe(Ⅱ)保持稳定并可以远距离迁移的关键.研究还表明,原儿茶酸、咖啡酸、没食子酸和龙胆酸对Fe(Ⅲ)有显着的还原作用,有利于沼泽区水体中的保持较高Fe(Ⅲ)和Fe(Ⅱ)浓度.哈尼泥炭中铁主要以活动态(可交换态、络合态和无定形态)为主,为铁的迁移、转化和循环奠定了基础.鉴于泥炭沼泽在全球的分布面积巨大以及亚铁对海洋生物有促进作用,酚酸对铁的作用机制对陆地系统向海洋输送生物可利用铁具有重要意义,并对碳循环、硫循环以及气候变化有重要影响.Abstract: The influence of dissolved organic matters as metal chelators on the bio-available iron input to the ocean has been widely reported by several studies. However, natural dissolved organic matters, especially the phenolics originated from peatlands and geochemical interactions with iron remains poorly understood. Hani peatland, as the national nature reserve in Jilin Province, is located in the central Longgang Mountain on the west side of Changbai Mountains. Physiochemical characteristics of water samples collected from rivers in Hani, including total dissolved iron, ferrous iron, dissolved organic carbon and pH etc., were detected in the field. Inner connections of these indexes were demonstrated through multivariate statistical analysis and simulation experiments on geochemical interactions between iron and phenolic acids were conducted in laboratory. Results show that total dissolved phenol plays an important role in the existence and transportation of ferrous iron. Ten phenolic acid, including protocatechuic acid, caffeic acid, gallic acid, gentisic acid, syringic acid, ferulic acid, p-hydroxybenzoic acid, p-coumaric acid, salicylic acid and vanillic acid, were detected by high performance liquid chromatography. Simulation experiments reveal that phenolics bearing either catechol or galloyl moiety groups (protocatechuic acid, caffeic acid and galllic acid) could chelate ferrous iron, which is the geochemical cause of high concentration of dissolved iron and is crucial for iron transport in peatland. Reducing action of phenolics to Fe(Ⅲ) is also responsible for maintaining high concentration of Fe(Ⅱ) and Fe(Ⅲ) in rivers drained from peatland. Considering the wide distribution of peatlands globally, the higher concentration of Fe in peatlands, and the enhancement of marine organisms by Fe, the complexation and reductive actions between iron and phenolics originated from peatlands are of important significance to global iron cycle coupled with other element cycles, such as carbon and sulfur, which can significantly influence global ecological balance.
-
Key words:
- Hani /
- peatland /
- phenolic acid /
- iron /
- environmental geology /
- geochemistry
-
表 1 铁与其他指标的相关性分析
Table 1. Correlation analysis of iron and various chemical indexes
氟离子 氯离子 硝酸根 硫酸根 亚铁 总铁 总酚 UV254 DOC 氟离子 1 0.570** -0.369 -0.401* 0.523** 0.531** 0.415* 0.566** 0.132 氯离子 1 -0.313 -0.209 0.480* 0.401* 0.312 0.353 0.360 硝酸根 1 0.378 -0.551** -0.604** -0.585** -0.714** -0.649** 硫酸根 1 -0.133 -0.741** -0.717** -0.636** -0.529** 亚铁 1 0.547** 0.489** 0.703** 0.354 总铁 1 0.923** 0.929** 0.706** 总酚 1 0.897** 0.773** UV254 1 0.709** DOC 1 注:**.在0.01水平(双侧)上显著相关;*.在0.05水平(双侧)上显著相关. 表 2 铁的理论形态和浓度(pH=8)
Table 2. Theoretical species and concentration of iron
理论形态 浓度(mol/L) Fe(OH)3- 9.76×10-24 Fe(OH)+ 3.83×10-20 Fe(OH)+2 1.17×10-24 Fe(OH)2+ 4.58×10-19 Fe2(OH)24+ 4.53×10-47 Fe(OH)3(aq) 4.95×10-19 Fe(OH)4- 4.64×10-20 Fe3(OH)45+ 3.00×10-64 Fe(OH)2(aq) 3.06×10-23 表 3 没食子酸、咖啡酸和原儿茶酸加入亚铁前后特征峰波长变化
Table 3. Changes in absorption spectra for gallic acid, caffeic acid and protocatechuic acid before and after addition of Fe2+
酚酸 特征峰波长(nm) 加入亚铁后特征峰波长(nm) 没食子酸 220,260 230,290 咖啡酸 230,290,310 285,/,330 原儿茶酸 250,289 300 注:“/”代表数值在检测限以下. -
Andjelkovi, M., van Camp, J., de Meulenaer, B., et al., 2006.Iron-Chelation Properties of Phenolic Acids Bearing Catechol and Galloyl Groups.Food Chemistry, 98(1):23-31.doi: 10.1016/j.foodchem.2005.05.044 Boyd, P.W., Jickells, T., Law, C.S., et al., 2007.Mesoscale Iron Enrichment Experiments 1993-2005:Synthesis and Future Directions Will Ocean Fertilization Work? Science, 315(5616):612-617.doi: 10.1126/science.1131669 Dwibedy, P., Dey, G.R., Naik, D.B., et al., 1999.Pulse Radiolysis Studies on Redox Reactions of Gallic Acid:One Electron Oxidation of Gallic Acid by Gallic Acid OH Adduct.Physical Chemistry Chemical Physics, 1(8):1915-1918.doi: 10.1039/A809147A Falkowski, P., Scholes, R.J., Boyle, E., et al., 2000.The Global Carbon Cycle:A Test of Our Knowledge of Earth as a System.Science, 290(5490):291-296.doi: 10.1126/science.290.5490.291 Fan, D.J., Ye, S.Y., Ding, X.G., et al., 2014.Authigenic Lepidocrocite and Greigite Particles in Aquatic Environment off the Yangtze River Estuary.Earth Science, 39(10):1464-1470. Gajewski, K., 2001.Sphagnum Peatland Distribution in North America and Eurasia during the Past 21 000 Years.Global Biogeochemical Cycles, 15(2):297-310.doi: 10.1029/2000GB001286 Graham, T.L., 1991.Flavonoid and Isoflavonoid Distribution in Developing Soybean Seedling Tissues and in Seed and Root Exudates.Plant Physiol, 95(2):594-603. doi: 10.1104/pp.95.2.594 Harwood, C.S., Parales, R.E., 1996.The b-Ketoadipate Pathway and the Biology of Self-Identity.Annual Review of Microbiology, 50(1):553-590.doi: 10.1146/annurev.micro.50.1.553 Huang, T., 2013.Peatland Archives of Holocene Volcanic Eruption Response to Paleoclimate in Northeast China (Dissertation).China University of Geosciences, Wuhan (in Chinese with English abstract). Jiang, M., Lü, X.G., Yang, Q., et al., 2006.Iron Biogeochemical Cycle and Its Environmental Effect in Wetlands.Acta Pedologica Sinica, 43(3):493-499 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-TRXB200603019.htm Jiang, S.J., Liu, Z.Y., 2002.The Meaning of UV254 as an Organic Matter Monitoring Parameter in Water Supply & Wastewater Treatment.Journal of Chongqing Jianzhu University, 24(2):61-65 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-JIAN200202014.htm Krachler, R., Krachler, R.F., von der Kammer, F., et al., 2010.Relevance of Peat-Draining Rivers for the Riverine Input of Dissolved Iron into the Ocean.Science of the Total Environment, 408(11):2402-2408.doi: 10.1016/j.scitotenv.2010.02.018 Liesack, W., Schnell, S., Revsbech, N.P., 2000.Microbiology of Flooded Rice Paddies.FEMS Microbiology Reviews, 24(5):625-645.doi: 10.1016/S0168-6445(00)00050-4 Liu, W.K., 2001.The Studies on Fe Provision Mechanisms and Biological Effects of Compound Iron Fertilizer.Agricultural University of Hebei, Baoding (in Chinese with English abstract). Martin, J.H., 1990.Glacial-Interglacial CO2 Change:The Iron Hypothesis.Paleoceanography, 5(1):1-13.doi: 10.1029/PA005i001p00001 Martin, J.H., Fitzwater, S.E., 1988.Iron Deficiency Limits Phytoplankton Growth in the North-East Pacific Subarctic.Nature, 331(6154):341-343.doi: 10.1038/331341a0 Martin, J.H., Gordon, R.M., Fitzwater, S.E., 1990.Iron in Antarctic Waters.Nature, 345(6271):156-158.doi: 10.1038/345156a0 Martin, J.H., Gordon, R.M., Fitzwater, S.E., 1991.The Case for Iron.Limnol.Oceanogr., 36:1793-1802.doi: 10.4319/lo.1991.36.8.1793 Matsunaga, K., Nishioka, J., Kuma, K., et al., 1998.Riverine Input of Bioavailable Iron Supporting Phytoplankton Growth in Kesennuma Bay(Japan).Water Research, 32(11):3436-3442.doi: 10.1016/S0043-1354(98)00113-4 Moran, J.F., Klucas, R.V., Grayer.R.J., et al., 1997.Complexes of Iron with Phenolic Compounds from Soybean Nodules and Other Legume Tissues:Prooxidant and Antioxidant Properties.Free Radical Biology and Medicine, 22(5):861-870. doi: 10.1016/S0891-5849(96)00426-1 Powell, R.T., Landing, W.M., Bauer, J.E., 1996.Colloidal Trace Metals, Organic Carbon and Nitrogen in a Southeastern U.S.Estuary.Marine Chemistry, 55(1-2):165-176.doi: 10.1016/S0304-4203(96)00054-0 Powell, R.T., Wilson-Finelli, A., 2003.Importance of Organic Fe Complexing Ligands in the Mississippi River Plume.Estuarine, Coastal and Shelf Science, 58(4):757-763.doi: 10.1016/S0272-7714(03)00182-3 Pracht, J., Boenigk, J., Isenbeck-Schröter, M., et al., 2001.Abiotic Fe (Ⅲ) Induced Mineralization of Phenolic Substances.Chemosphere, 44(4):613-619.doi: 10.1016/S0045-6535(00)00490-2 Qiao, S.Y., 1993.A Preliminary Study on Hani Peat-Mire in the West Part of the Changbai Mountain.Scientia Geographica Sinica, 13(3):279-287(in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-DLKX199303011.htm Raiswell, R., 2006.Towards a Global Highly Reactive Iron Cycle.Journal of Geochemical Exploration, 88(1):436-439.doi: 10.1016/j.gexplo.2005.08.098 Roden, E.E., Wetzel, R.G., 2002.Kinetics of Microbial Fe(Ⅲ) Oxide Reduction in Freshwater Wetland Sediments.Limnology and Oceanography, 47(1):198-211.doi: 10.4319/lo.2002.47.1.0198 Rose, A.L., Waite, T.D., 2003.Kinetics of Iron Complexation by Dissolved Natural Organic Matter in Coastal Waters.Marine Chemistry, 84(1-2):85-103.Doi: 10.1016/S0304-4203(03)00113-0 Saitoh, Y., Nakatsuka, T., Kuma, K., et al., 2008.Processes Influencing Iron Distribution in the Coastal Waters of the Tsugaru Strait, Japan.Journal of Oceanography, 64(6):815-830.doi: 10.1007/s10872-008-0068-3 Strli, M., Radovi, T., Kolar, J., et al., 2002.Anti-and Prooxidative Properties of Gallic Acid in Fenton-Type Systems.Journal of Agricultural and Food Chemistry, 50(22):6313-6317.doi: 10.1021/jf025636j Sun, X.T., 2011.Effects of Hydrothermal Changes on Phenolic Compounds and Phenol Oxidase Activities in Peatlands (Dissertation).China University of Geosciences, Wuhan (in Chinese with English abstract). Turner, S.M., Nightingale, P.D., Spokes, L.J., et al., 1996.Increased Dimethyl Sulphide Concentrations in Sea Water from In-Situ Iron Enrichment.Nature, 383(6600):513-517.doi: 10.1038/383513a0 Wan, X., Xiang, W., Wu, Y., et al., 2013a.Causes of High Concentration Dissolved Iron in Plateau Peatland.Environmental Science & Technology, 36(11):7-11.doi: 10.3969/j.issn.1003-6504.2013.11.002 Wan, X., Xiang, W., Yu, S., 2013b.Determination of Phenols from Peatland Water by Solid Phase Extraction and High Performance Liquid Chromatography.Chinese Journal of Analysis Laboratory, 32(10):15-19.doi: 10.13595/j.cnki.issn1000-0720.2013.0259 Windom, H.L., Niencheski, L.F., Smith Jr, R.G., 1999.Biogeochemistry of Nutrients and Trace Metals in the Estuarine Region of the Patos Lagoon (Brazil).Estuarine, Coastal and Shelf Science, 48(1):113-123.Doi: 10.1006/ecss.1998.0410 Witter, A.E., Luther Ⅲ, G.W., 1998.Variation in Fe-Organic Complexation with Depth in the Northwestern Atlantic Ocean as Determined Using a Kinetic Approach.Marine Chemistry, 62(3-4):241-258.doi: 10.1016/S0304-4203(98)00044-9 Xu, S.L., Duan, W.H., Liu, S.J., et al., 1986.A Study on the Ferrous Iron Oxidized by the Air in Aqueous Solution—Ⅰ.The Effect of pH Value on the Oxidizing Rate and the Catalytic Mechanism of the "Screen Effect" on Hydrated Ferrous Ion Destroyed by the Hydrolytic Product of Ferric Ion.Journal of Yunnan University, 8(2):191-197 (in Chinese with English abstract). Zhao, G.M., Chen, B., Wang, L., et al., 2014.Sedimentary Environmental Partitioning of Holocene Strata and Assessment of Carbon Burial Rate of Various Paleo-Environments in the Yellow River Delta.Earth Science, 39(4):451-461. Zou, Y.C., Jiang, M., 2008.Comparison of Analysis and Determination Methods of Iron in Wetland Soil.Wetland Science, 6(2):136-141(in Chinese with English abstract). 范德江, 陈彬, 王亮, 等, 2014.长江口外悬浮颗粒物中自生纤铁矿和胶黄铁矿.地球科学, 39(10):1464-1470. http://earth-science.net/WebPage/Article.aspx?id=2947 黄庭, 2013. 东北泥炭记录的全新世火山喷发事件及其古气候响应研究(博士学位论文). 武汉: 中国地质大学. 姜明, 吕宪国, 杨青, 等, 2006.湿地铁的生物地球化学循环及其环境效应.土壤学报, 43(3):493-499. doi: 10.11766/trxb200412270320 蒋绍阶, 刘宗源, 2002.UV254作为水处理中有机物控制指标的意义.重庆建筑大学学报, 24(2):61-65. 刘文科, 2001. 复混铁肥的供铁机理及其生物效应研究(硕士学位论文). 保定: 河北农业大学. 乔石英, 1993.长白山西麓哈尼泥炭沼泽初探.地理科学, 13(3):279-287. http://www.cnki.com.cn/Article/CJFDTOTAL-DLKX199303011.htm 孙兴庭, 2011. 水热条件变化对泥炭沼泽中酚类物质和酚氧化酶的影响及其意义(硕士学位论文). 武汉: 中国地质大学. 万翔, 向武, 邬钰, 等, 2013a.高原泥炭沼泽区高浓度溶解性铁的成因研究.环境科学与技术, 36(11):7-11. http://www.cnki.com.cn/Article/CJFDTOTAL-FJKS201311002.htm 万翔, 向武, 于桑, 等, 2013b.固相萃取-高效液相色谱法同时测定泥炭沼泽源水体中9种酚类物质.分析试验室, 32(10):15-19. http://www.cnki.com.cn/Article/CJFDTOTAL-FXSY201310006.htm 徐绍龄, 段维恒, 刘时杰, 等.空气氧化水溶液中亚铁离子的研究——Ⅰ.溶液pH值对氧化速率的影响及铁的水解产物破坏水合亚铁离子"遮蔽效应"的催化机理.云南大学学报(自然科学版), 8(2):191-197. http://www.cnki.com.cn/Article/CJFDTOTAL-YNDZ198602015.htm 赵广明, 叶思源, 丁喜桂, 等, 2014.黄河三角洲全新世以来沉积环境的划分及各环境中碳埋藏速率的评价.地球科学, 39(4):451-461. http://earth-science.net/WebPage/Article.aspx?id=2854 邹元春, 姜明, 2008.湿地土壤铁的分析测定方法比较.湿地科学, 6(2):136-141. http://www.cnki.com.cn/Article/CJFDTOTAL-KXSD200802007.htm -









 下载:
下载: