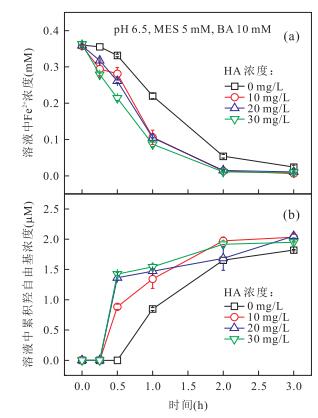
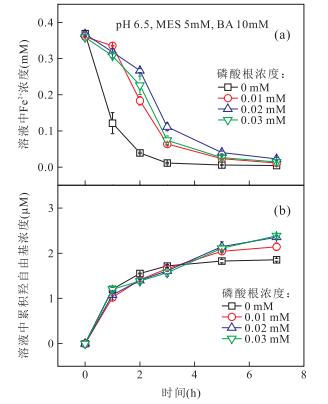
|
Baltpurvins, K.A., Burns, R.C., Lawrance, G.A., et al., 1997.Effect of Ca2+, Mg2+, and Anion Type on the Aging of Iron(Ⅲ) Hydroxide Precipitates.Environmental Science & Technology, 31(4):1024-1032.doi: 10.1021/es960498y |
|
Chen, Y.M., Chen, Z.H., Yu, K.B., 2016.Heterogeneity and Water Prevention of Karst Water System in Metal Mine Areas in Southern China:A Case Study of Makeng Iron Mine, Fujian Province.Earth Science, 41(4):692-700(in Chinese with English abstract). http://www.en.cnki.com.cn/Article_en/CJFDTotal-DQKX201604015.htm |
|
Deng, Y.M., Wang, Y.X., Li, H.J., et al., 2015.Seasonal Variation of Arsenic Speciation in Shallow Groundwater from Endemic Arsenicosis Area in Jianghan Plain.Earth Science, 40(11):1876-1886(in Chinese with English abstract). https://www.researchgate.net/publication/288228393_Seasonal_variation_of_arsenic_speciation_in_shallow_groundwater_from_endemic_arsenicosis_area_in_Jianghan_Plain |
|
Duan, Y.H., Gan, Y.Q., Guo, X.X., et al., 2014.Water Chemical Characteristics and Arsenic Enrichment Factor Analysis at High Arsenic Groundwater Monitoring Field of Jianghan Plain.Geological Science and Technology Information, 33(2):140-147(in Chinese with English abstract). |
|
Goti, M., Musi, S., Popovi, S., et al., 2008.Investigation of Factors Influencing the Precipitation of Iron Oxides from Fe(Ⅱ) Containing Solutions.Croatica Chemica Acta, 81(4):569-578. https://www.researchgate.net/publication/288079329_Investigation_of_factors_influencing_the_precipitation_of_iron_oxides_from_FeII_containing_solutions |
|
Hug, S.J., Leupin, O., 2003.Iron-Catalyzed Oxidation of Arsenic(Ⅲ) by Oxygen and by Hydrogen Peroxide:pH-Dependent Formation of Oxidants in the Fenton Reaction.Environmental Science & Technology, 37(12):2734-2742.doi: 10.1021/es026208x |
|
Keenan, C.R., Sedlak, D.L., 2008.Ligand-Enhanced Reactive Oxidant Generation by Nanoparticle Zero-Valent Iron and Oxygen.Environmental Science & Technology, 42(18):6936-6941.doi: 10.1021/es801438 |
|
Komadel, P., Stucki, J.W., 1988.Quantitative Assay of Minerals for Fe2+ and Fe3+ Using 1.10-Phenathroline:Ⅲ.A Rapid Photochemical Method.Clays and Clay Minerals, 36(4):379-381.doi: 10.1346/CCMN.1988.0360415 |
|
Koppenol, W.H., 2001.The Haber-Weiss Cycle-70 Years Later.Redox Report, 6(4):229-234.doi: 10.1179/135100001101536373 |
|
Lee, C.H., Keenan, C.R., Sedlak, D.L., 2008.Polyoxometalate-Enhanced Oxidation of Organic Compounds by Nanoparticulate Zero-Valent Iron and Ferrous Ion in the Presence of Oxygen.Environmental Science & Technology, 42(13):4921-4926.doi: 10.1021/es800317j |
|
Li, G.B., Liu, C., 1989.Eliminating Iron and Manganese from Groundwater(2nd Edition).China Architecture & Building Press, Beijing (in Chinese). |
|
Liang, L.Y., McNabb, J.A., Paulk, J.M., et al., 1993.Kinetics of Iron(Ⅱ) Oxygenation at Low Partial Pressure of Oxygen in the Presence of Natural Organic Matter.Environmental Science & Technology, 27(9):1864-1870.doi: 10.1021/es00046a014 |
|
Liao, P., Li, W.L., Wang, D.J., et al., 2017.Effect of Reduced Humic Acid on the Transport of Ferrihydrite Nanoparticles under Anoxic Conditions.Water Research, 109:347-357.doi: 10.1016/j.watres.2016.11.069 |
|
Mopper, K., Zhou, X., 1990.Hydroxyl Radical Photoproduction in the Sea and Its Potential Impact on Marine Processes.Science, 250(4981):661-664.doi: 10.1126/science.250.4981.661 |
|
Page, S.E., Kling, G.W., Sander, M., et al., 2013.Dark Formation of Hydroxyl Radicals in Arctic Soil and Surface Waters.Environmental Science & Technology, 47(22):12860-12867.doi: 10.1021/es4033265 |
|
Page, S.E., Sander, M., Arnold, W.A., et al., 2012.Hydroxyl Radical Formation upon Oxidation of Reduced Humic Acids by Oxygen in the Dark.Environmental Science & Technology, 46(3):1590-1597.doi: 10.1021/es203836f |
|
Pang, S.Y., Jiang, J., Ma, J., 2011.Oxidation of Sulfoxides and Arsenic(Ⅲ) in Corrosion of Nanoscale Zero Valent Iron by Oxygen:Evidence against Ferryl Ions (Fe(Ⅳ)) as Active Intermediates in Fenton Reaction.Environmental Science & Technology, 45(1):307-312.doi: 10.1021/es102401d |
|
Rose, J., Flank, A.M., Masion, A., et al., 1997.Nucleation and Growth Mechanisms of Fe Oxyhydroxide in the Presence of PO4 Ions.2.P K-Edge EXAFS Study.Langmuir, 13(6):1827-1834.doi: 10.1021/la961039d |
|
Rose, J., Manceau, A., Bottero, J.Y., et al., 1996.Nucleation and Growth Mechanisms of Fe Oxyhydroxide in the Presence of PO4 Ions.1.Fe K-Edge EXAFS Study.Langmuir, 12(26):6701-6707.doi: 10.1021/la9606299 |
|
Sharma, P., Ofner, J., Kappler, A., 2010.Formation of Binary and Ternary Colloids and Dissolved Complexes of Organic Matter, Fe and As.Environmental Science & Technology, 44(12):4479-4485.doi: 10.1021/es100066s |
|
Stumm, W., Sulzburger, B., 1992.The Cycling of Iron in Natural Environments:Considerations Based on Laboratory Studies of Heterogeneous Redox Processes.Geochimica et Cosmochimica Acta, 56(8):3233-3257.doi: 10.1016/0016-7037(92)90301-X |
|
Tong, M., Yuan, S.H., Ma, S.C., et al., 2016.Production of Abundant Hydroxyl Radicals from Oxygenation of Subsurface Sediments.Environmental Science & Technology, 50(1):214-221.doi: 10.1021/acs.est.5b04323 |
|
Tryk, D.A., Fujishima, A., Honda, K., 2000.Recent Topics in Photoelectrochemistry:Achievements and Future Prospects.Electrochimica Acta, 45(15-16):2363-2376.doi: 10.1016/S0013-4686(00)00337-6 |
|
Zhang, N.D., Zheng W., 2001.The Application of Hydroxyl Radical in Wasterwater Treatment.Journal of Harbin University of Commerce (Natural Sciences Edition), 17(3):22-24, 28(in Chinese with English abstract). doi: 10.1080/10643389.2010.507698?queryID=%24%7BresultBean.queryID%7D |
|
Zhang, R.Q., Liang, X., Jin, M.G., et al., 2001.General Hydrogeology.6th Edition.Geological Publishing House, Beijing, 64 (in Chinese). |
|
Zhu, J., Zhang, P., Yuan, S.H., et al., 2017.Production of Hydroxyl Radicals from Oxygenation of Simulated AMD Due to CaCO3-Induced pH Increase.Water Research, 111:118-126.doi: 10.1016/j.watres.2016.12.048 |
|
陈彦美, 陈植华, 於开炳, 2016.南方岩溶金属矿区地下水非均质性及防治水意义:以福建马坑铁矿为例.地球科学, 41(4):692-700. http://www.earth-science.net/WebPage/Article.aspx?id=3286 |
|
邓娅敏, 王焰新, 李慧娟, 等, 2015.江汉平原砷中毒病区地下水砷形态季节性变化特征.地球科学, 40(11):1876-1886. http://www.earth-science.net/WebPage/Article.aspx?id=3194 |
|
段艳华, 甘义群, 郭欣欣, 等, 2014.江汉平原高砷地下水监测场水化学特征及砷富集影响因素分析.地质科技情报, 33(2):140-147. http://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201402024.htm |
|
李圭白, 刘超, 1989.地下水除铁除锰(第2版).北京:中国建筑工业出版社. |
|
张乃东, 郑威, 2001.羟自由基·OH在水处理中的应用.哈尔滨商业大学学报(自然科学版), 17(3):22-24, 28. http://www.cnki.com.cn/Article/CJFDTOTAL-HLJS200103006.htm |
|
张人权, 梁杏, 靳孟贵, 等, 2010.水文地质学基础(第六版).北京:地质出版社, 64. |








 下载:
下载: