Nanomineral-Aqueous Solution Interfacial Processes
-
摘要: 在地球环境中普遍存在的纳米矿物-水溶液界面对许多基本的地球化学过程都至关重要,因而是纳米地球化学的前沿核心研究领域.简要介绍了纳米矿物-水溶液界面领域的基本概念和近期研究进展.举例描述了纳米矿物团聚、吸附、溶解和化学反应等几个相互关联的主要过程,具体阐述了纳米矿物自身特征(如组成、结构、尺寸、形貌、表面保护剂等)以及环境介质条件(如pH、离子强度、化学反应物质、天然有机质浓度和组成、微生物、光辐射等)对纳米矿物-水溶液界面过程的影响规律和微观机制.针对本领域发展面临的机遇和挑战,为未来的研究方向提出了一些设想和建议.Abstract: Nanomineral-aqueous solution interface is ubiquitous in the earth environment, and is of critical importance to many fundamental geochemical processes. The study on nanomineral-aqueons interfaces is therefore at the forefront of nanogeochemistry. In this paper, it briefly introduces the basic concepts and recent research progresses in the field of nanomineral-aqueons interfaces, and specifically illustrates major interfacial processes including aggregation, adsorption, dissolution and chemical reaction of nanominerals. The effects and microscopic mechanisms of nanomineral characteristics (such as composition, structure, size, morphology, surface protection layer, etc.) and environmental media conditions (including pH, ionic strength, chemical reaction substances, NOM concentration and composition, microorganism, light radiation, etc.) on the interfacial processes are discussed in detail. In view of the opportunities and challenges presented in this field, some suggestions for future research directions are put forward.
-
Key words:
- nanogeochemisty /
- mineral-aqueous solution interface /
- nanomineral /
- sorption /
- dissolution /
- aggregation
-
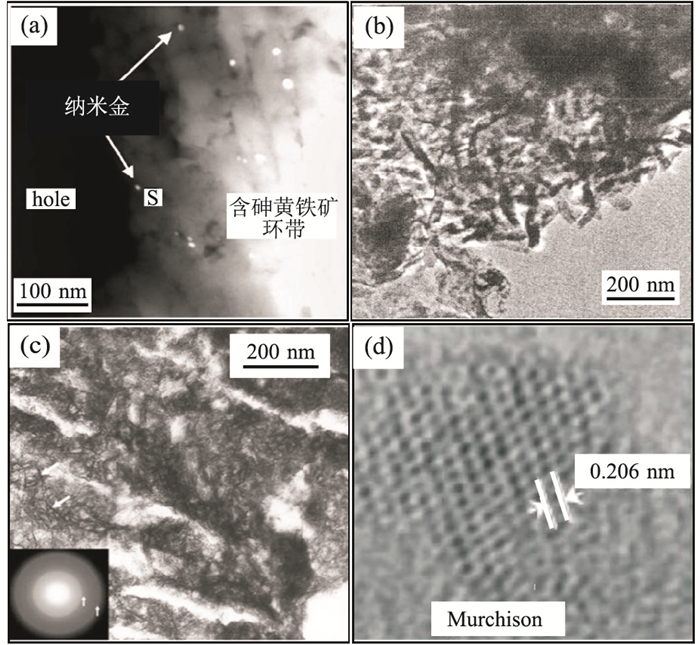
图 1 自然界存在的纳米矿物的透射电镜照片
a.卡林型金矿床含砷黄铁矿中浸染状分布的金纳米颗粒(Reich et al., 2005);b.洛川黄土中的纳米棒状方解石(陈天虎等, 2005);c.位于Miles Crossing河床中的6-线水铁矿(Hochella et al., 2005);d.Murchison陨石中平均粒径3 nm的纳米金刚石(Dai et al., 2002)
Fig. 1. TEM images of naturally occurring nanominerals and mineral nanoparticles
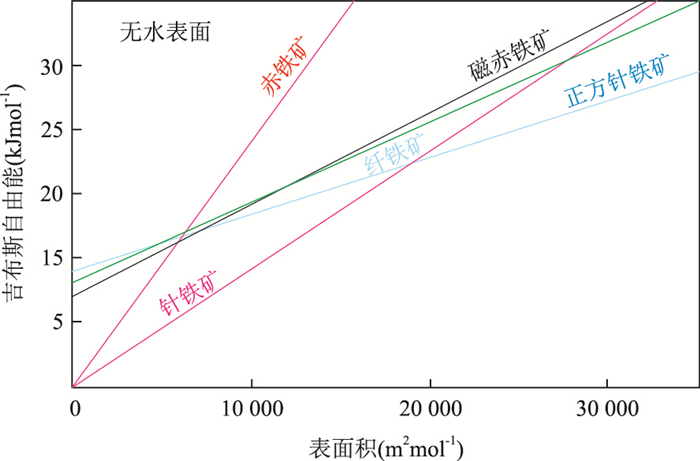
图 2 多种铁氧化物和羟基氧化物的吉布斯自由能随比表面积(或粒径)的变化
Fig. 2. Gibbs free energy of various iron oxides and oxyhydroxides as a function of specific surface area (or particle size)
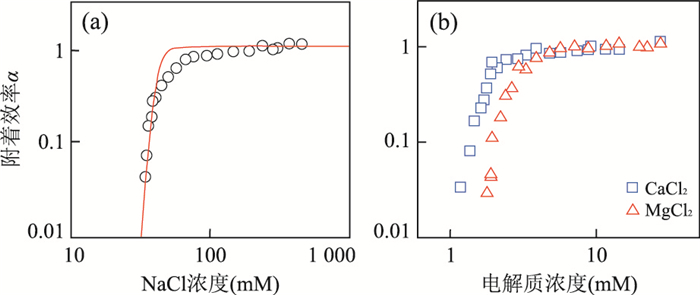
图 4 柠檬酸盐保护的纳米银的附着系数随不同离子浓度的变化(pH=7.0)
据Huynh and Chen(2011).a.NaCl;b.CaCl2和MgCl2
Fig. 4. Attachment efficiencies of citrate-coated AgNPs as functions of NaCl (a) and CaCl2 and MgCl2 (b) concentrations at pH=7.0
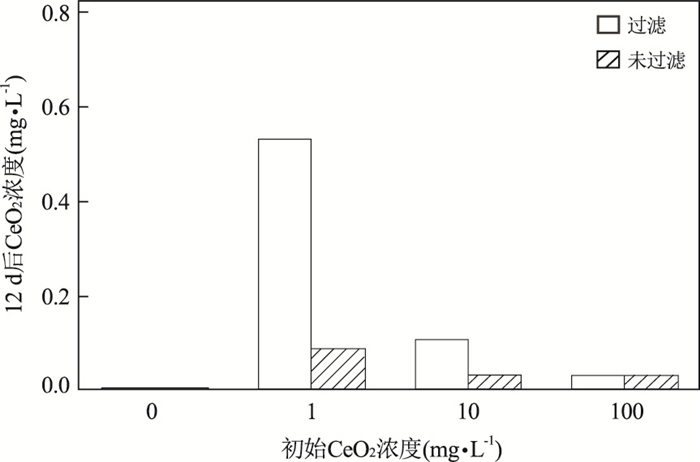
图 6 CeO2纳米粒子在过滤和未过滤的莱茵河、默兹河水样中沉降12 d后的平均剩余浓度
Fig. 6. Residual concentrations of CeO2 nanoparticles after 12 d of settling in filtered and unfiltered river water, average of concentration in Rhine and Meuse Rivers
图 7 Ag和TiO2纳米粒子与蒙脱石吸附作用示意图
Fig. 7. Schematic diagram of sorption of AgNPs and TiO2 NPs on montmorillonite
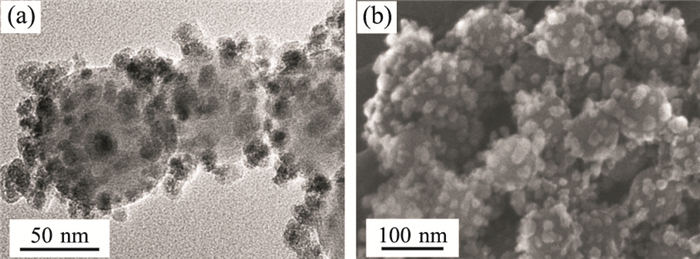
图 8 aSNPs和cMNPs异相共团聚的TEM照片(a)和SEM照片(b)
Fig. 8. TEM (a) and SEM (b) images of the heteroaggregates formed between the aSNPs and the cMNPs
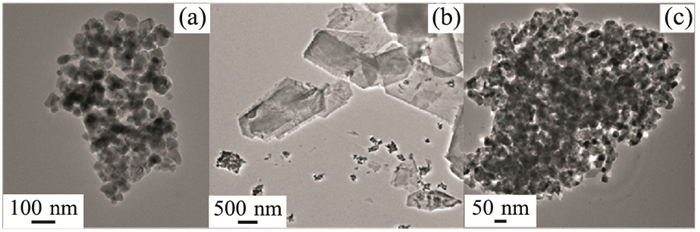
图 9 原始状态ZnO纳米粒子TEM照片(a)以及在pH=6(b)和pH=8(c)的磷酸盐溶液(150 mg L-1)中熟化72 h后TEM照片
Fig. 9. TEM images of pristine ZnO NPs (a) and MNMs aged in 150 mg L-1 phosphate concentration for 72 h at pH=6 (b) and at pH=8 (c)
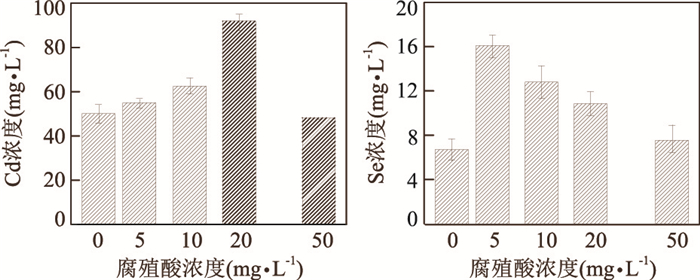
图 10 PDDA保护的CdSe/ZnS量子点在紫外光辐射3 h后溶出离子Cd和Se的浓度随腐殖酸浓度的变化
Fig. 10. Cd and Se release of PDDA-coated QDs as a function of HA concentration after 3 h UV irradiation
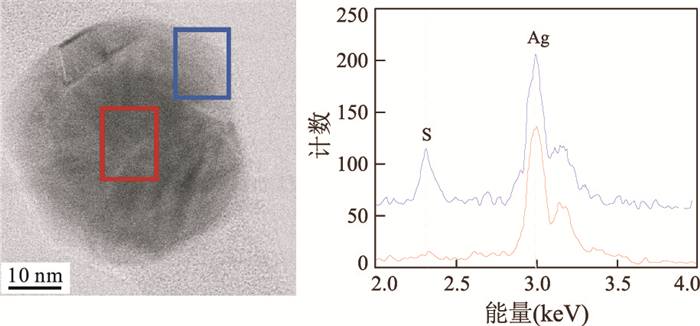
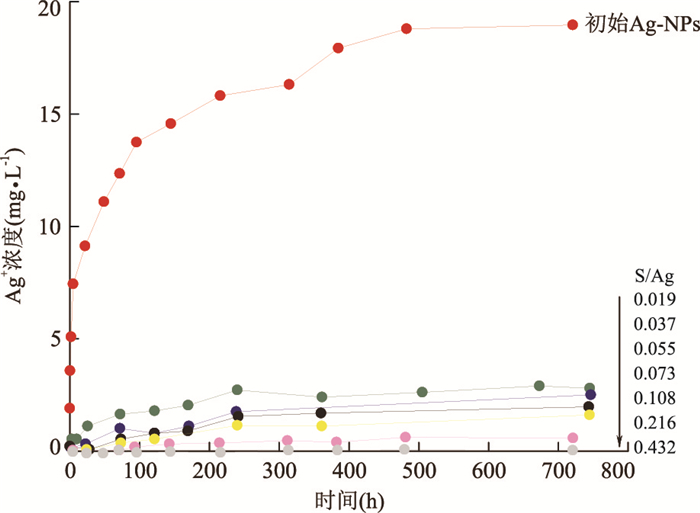
图 11 废水实验中收集的AgNP的相差明场扫描透射电镜(STEM)照片(a)和EDX图谱指示S/Ag比例随空间的变化(b)
据Kaegi et al.(2013).b.蓝色图谱指示左图篮框区域,红色图谱指示左图红色区域
Fig. 11. Phase contrast bright field scanning transmission electron microscopy (STEM) image of an AgNP collected from the sewer batch experiments (a) and EDX spectra revealing spatial variations in the S/Ag ratios (b)
图 12 纳米银与浓度递增的Na2S反应前后溶解速率的变化
据Levard et al.(2011).在0.01 mol/L NaNO3,pH=7溶解速率实验中初始纳米银浓度为1 000 mg·L-1
Fig. 12. Dissolution rate measurements of Ag-NPs before and after reaction with increasing concentrations of aqueous Na2S
-
Alivisatos, A.P., 1996.Semiconductor Clusters, Nanocrystals, and Quantum Dots.Science, 271(5251):933-937. https://doi.org/10.1126/science.271.5251.933 Amde, M., Liu, J.F., Tan, Z.Q., et al., 2017.Transformation and Bioavailability of Metal Oxide Nanoparticles in Aquatic and Terrestrial Environments:A Review.Environmental Pollution, 230:250-267. https://doi.org/10.1016/j.envpol.2017.06.064 Arvidsson, R., Molander, S., Sanden, B.A., et al., 2011.Challenges in Exposure Modeling of Nanoparticles in Aquatic Environments.Human and Ecological Risk Assessment, 17(1):245-262. https://doi.org/10.1080/10807039.2011.538639 Banfield, J.F., Zhang, H., 2001.Nanoparticles in the Environment.Reviews in Mineralogy and Geochemistry, 44(1):1-58. doi: 10.2138/rmg.2001.44.01 Basu, S., Ghosh, S.K., Kundu, S., et al., 2007.Biomolecule Induced Nanoparticle Aggregation:Effect of Particle Size on Interparticle Coupling.Journal of Colloid and Interface Science, 313(2):724-734. https://doi.org/10.1016/j.jcis.2007.04.069 Batley, G.E., Kirby, J.K., McLaughlin, M.J., 2013.Fate and Risks of Nanomaterials in Aquatic and Terrestrial Environments.Accounts of Chemical Research, 46(3):854-862. https://doi.org/10.1021/ar2003368 Baumann, J., Koeser, J., Arndt, D., et al., 2014.The Coating Makes the Difference:Acute Effects of Iron Oxide Nanoparticles on Daphnia Magna.Science of the Total Environment, 484:176-184. https://doi.org/10.1016/j.scitotenv.2014.03.023 Bian, S.W., Mudunkotuwa, I.A., Rupasinghe, T., et al., 2011.Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments:Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid.Langmuir, 27(10):6059-6068. https://doi.org/10.1021/la200570n Bondarenko, O., Ivask, A., Kakinen, A., et al., 2013.Particle-Cell Contact Enhances Antibacterial Activity of Silver Nanoparticles.PLoS One, 8(5):e64060. https://doi.org/10.1371/journal.pone.0064060 Borm, P.J.A., Robbins, D., Haubold, S., et al., 2006.The Potential Risks of Nanomaterials:A Review Carried out for ECETOC.Particle and Fibre Toxicology, 3:11. https://doi.org/10.1186/1743-8977-3-11 Brant, J.A., Labille, J., Robichaud, C.O., et al., 2007.Fullerol Cluster Formation in Aqueous Solutions:Implications for Environmental Release.Journal of Colloid and Interface Science, 314(1):281-288. https://doi.org/10.1016/j.jcis.2007.05.020 Brantley, S.L., Kubicki, J.D., White, A.F., 2008.Kinetics of Water-Rock Interaction.Springer, New York, 73-101. Brown, G.E.Jr., Calas, G., 2012.Mineral-Aqueous Solution Interfaces and Their Impact on the Environment.Geochemical Perspectives, 1(4-5):483-742. https://doi.org/10.7185/geochempersp.1.4 Brunet, L., Lyon, D.Y., Hotze, E.M., et al., 2009.Comparative Photoactivity and Antibacterial Properties of C-60 Fullerenes and Titanium Dioxide Nanoparticles.Environmental Science & Technology, 43(12):4355-4360. https://doi.org/10.1021/es803093t Buffle, J., Leppard, G.G., 1995.Characterization of Aquatic Colloids and Macromolecules.1.Structure and Behavior of Colloidal Material.Environmental Science & Technology, 29(9):2169-2175. https://doi.org/10.1021/es00009a004 Buffle, J., Wilkinson, K.J., Stoll, S., et al., 1998.A Generalized Description of Aquatic Colloidal Interactions:The Three-Colloidal Component Approach.Environmental Science & Technology, 32(19):2887-2899. https://doi.org/10.1021/es980217h Burrows, N.D., Hale, C.R.H., Penn, R.L., 2012.Effect of Ionic Strength on the Kinetics of Crystal Growth by Oriented Aggregation.Crystal Growth & Design, 12(10):4787-4797. https://doi.org/10.1021/cg3004849 Cerbelaud, M., Videcoq, A., Abelard, P., et al., 2008.Heteroaggregation between Al2O3 Submicrometer Particles and SiO2 Nanoparticles:Experiment and Simulation.Langmuir, 24(7):3001-3008. https://doi.org/10.1021/la702104u Charlet, L., Morin, G., Rose, J., et al., 2011.Reactivity at (Nano) particle-Water Interfaces, Redox Processes, and Arsenic Transport in the Environment.Comptes Rendus Geoscience, 343(2-3):123-139. https://doi.org/10.1016/j.crte.2010.11.005 Chen, K.L., Elimelech, M., 2007.Influence of Humic Acid on the Aggregation Kinetics of Fullerene (C-60) Nanoparticles in Monovalent and Divalent Electrolyte Solutions.Journal of Colloid and Interface Science, 309(1):126-134. https://doi.org/10.1016/j.jcis.2007.01.074 Chen, T.H., Chen, J., Ji, J.F., et al., 2005.Nanometer-Scale Investigation on the Loess of Luochuan:Nano-rod Calcite.Geological Review, 51(6):713-718, 741-742 (in Chinese with English abstract). https://www.deepdyve.com/lp/elsevier/morphological-characters-and-multi-element-isotopic-signatures-of-ZWRFrPAU0s Cheng, D., Liao, P., Yuan, S.H., 2016.Effect of FeS Colloids on Desorption of As (Ⅴ) Adsorbed on Ferric Iron.Earth Science, 41(2):325-330 (in Chinese with English abstract). http://www.en.cnki.com.cn/Article_en/CJFDTotal-DQKX201602012.htm Cho, M., Chung, H., Choi, W., et al., 2004.Linear Correlation between Inactivation of E-Coli and OH Radical Concentration in TiO2 Photocatalytic Disinfection.Water Research, 38(4):1069-1077. https://doi.org/10.1016/j.watres.2003.10.029 Conway, J.R., Hanna, S.K., Lenihan, H.S., et al., 2014.Effects and Implications of Trophic Transfer and Accumulation of CeO2 Nanoparticles in a Marine Mussel.Environmental Science & Technology, 48(3):1517-1524. https://doi.org/10.1021/es404549u Dai, Z.R., Bradley, J.P., Joswiak, D.J., et al., 2002.Possible In Situ Formation of Meteoritic Nanodiamonds in the Early Solar System.Nature, 418(6894):157-159. https://doi.org/10.1038/nature00897 Daou, T.J., Begin-Colin, S., Greneche, J.M., et al., 2007.Phosphate Adsorption Properties of Magnetite-Based Nanoparticles.Chemistry of Materials, 19(18):4494-4505. https://doi.org/10.1021/cm071046v Derjaguin, B.V., Landau, L., 1941.Theory of the Stability of Strongly Charged Lyophobic Sols and the Adhesion of Strongly Charged Particles in Solutions of Electrolytes.Acta Physicochim.URSS, 14(1):633-662. http://www.doc88.com/p-3187637119402.html Diegoli, S., Manciulea, A.L., Begum, S., et al., 2008.Interaction between Manufactured Gold Nanoparticles and Naturally Occurring Organic Macromolecules.Science of the Total Environment, 402(1):51-61. https://doi.org/10.1016/j.scitotenv.2008.04.023 Diegoli, S., Mendes, P.M., Baguley, E.R., et al., 2006.pH-dependent Gold Nanoparticle Self-Organization on Functionalized Si/SiO2 Surfaces.Journal of Experimental Nanoscience, 1(3):333-353. https://doi.org/10.1080/17458080600778644 Dušak, P., Mertelj, A., Kralj, S., et al., 2015.Controlled Heteroaggregation of Two Types of Nanoparticles in an Aqueous Suspension.Journal of Colloid and Interface Science, 438:235-243. https://doi.org/10.1016/j.jcis.2014.09.086 Ellis, L.J.A., Valsami-Jones, E., Lead, J.R., et al., 2016.Impact of Surface Coating and Environmental Conditions on the Fate and Transport of Silver Nanoparticles in the Aquatic Environment.Science of the Total Environment, 568:95-106. https://doi.org/10.1016/j.scitotenv.2016.05.199 Feng, L., Cao, M., Ma, X., et al., 2012.Superparamagnetic High-Surface-Area Fe3O4 Nanoparticles as Adsorbents for Arsenic Removal.Journal of Hazardous Materials, 217:439-446. https://doi.org/10.1016/j.jhazmat.2012.03.073 French, R.A., Jacobson, A.R., Kim, B., et al., 2008.Influence of Ionic Strength, pH, and Cation Valence on Aggregation Kinetics of TiO2 Nanoparticles.Geochimica et Cosmochimica Acta, 72(2):A283-A283. Fu, Y., Nie, X., Qin, Z., et al., 2017.Effect of Particle Size and Pyrite Oxidation on the Sorption of Gold Nanoparticles on the Surface of Pyrite.Journal of Nanoscience and Nanotechnology, 17(9):6367-6376. doi: 10.1166/jnn.2017.14417 Garcia-Perez, P., Pagnoux, C., Rossignol, F., et al., 2006.Heterocoagulation between SiO2 Nanoparticles and Al2O3 Submicronparticles; Influence of the Background Electrolyte.Colloids and Surfaces A-Physicochemical and Engineering Aspects, 281(1-3):58-66. https://doi.org/10.1016/j.colsurfa.2006.02.018 Garner, K.L., Keller, A.A., 2014.Emerging Patterns for Engineered Nanomaterials in the Environment:A Review of Fate and Toxicity Studies.Journal of Nanoparticle Research, 16(8). https://doi.org/10.1007/s11051-014-2503-2 Gilbert, B., Huang, F., Zhang, H.Z., et al., 2004.Nanoparticles:Strained and Stiff.Science, 305(5684):651-654. https://doi.org/10.1126/science.1098454 Gogos, A., Thalmann, B., Voegelin, A., et al., 2017.Sulfidation Kinetics of Copper Oxide Nanoparticles.Environmental Science-Nano, 4(8):1733-1741. https://doi.org/10.1039/c7en00309a He, H.P., Guo, J.G., Xie, X.D., et al., 1999.Experimental Studies on the Selective Adsorption of Cu2+, Pb2+, Zn2+, Cd2+, Cr3+ Ions on Montmorillonite, Illite and Kaolinite and the Influence of Medium Conditions.Acta Mineralogica Sinica, 19 (2):231-235 (in Chinese with English abstract). He, H.P., Guo, J.G., Zhu, J.X., et al., 2001.An Experimental Study of Adsorption Capacity of Montmorillonite, Kaolinite and Illite for Heavy Metals.Acta Petrologica et Mineralogica, 20(4):573-578 (in Chinese with English abstract). He, Y.T., Wan, J., Tokunaga, T., 2008.Kinetic Stability of Hematite Nanoparticles:The Effect of Particle Sizes.Journal of Nanoparticle Research, 10(2):321-332. https://doi.org/10.1007/s11051-007-9255-1 Herrington, R.J., Wilkinson, J.J., 1993.Colloidal Gold and Silica in Mesothermal Vein Systems.Geology, 21(6):539-542.https://doi.org/10.1130/0091-7613(1993)021 <0539:cgasim>2.3.co; 2 doi: 10.1130/0091-7613(1993)021<0539:cgasim>2.3.co;2 Hochella, M.F., 2002.Nanoscience and Technology:The Next Revolution in the Earth Sciences.Earth and Planetary Science Letters, 203(2):593-605. doi: 10.1016/S0012-821X(02)00818-X Hochella, M. F., Aruguete, D., Kim, B., et al., 2012. Nature's Nanostructures. Pan Stanford Publishing Pte. Ltd., Singpore. Hochella, M.F., Kasama, T., Putnis, A., et al., 2005.Environmentally Important, Poorly Crystalline Fe/Mn Hydrous Oxides:Ferrihydrite and a Possibly New Vernadite-Like Mineral from the Clark Fork River Superfund Complex.American Mineralogist, 90(4):718-724. https://doi.org/10.2138/am.2005.1591 Hochella, M.F.Jr., Lower, S.K., Maurice, P.A., et al., 2008.Nanominerals, Mineral Nanoparticles, and Earth Systems.Science, 319(5870):1631-1635. https://doi.org/10.1126/science.1141134 Hoek, E.M.V., Agarwal, G.K., 2006.Extended DLVO Interactions between Spherical Particles and Rough Surfaces.Journal of Colloid and Interface Science, 298(1):50-58. https://doi.org/10.1016/j.jcis.2005.12.031 Horev-Azaria, L., Baldi, G., Beno, D., et al., 2013.Predictive Toxicology of Cobalt Ferrite Nanoparticles:Comparative In-Vitro Study of Different Cellular Models Using Methods of Knowledge Discovery from Data.Particle and Fibre Toxicology, 10:32. https://doi.org/10.1186/1743-8977-10-32 Hotze, E.M., Phenrat, T., Lowry, G.V., 2010.Nanoparticle Aggregation:Challenges to Understanding Transport and Reactivity in the Environment.Journal of Environmental Quality, 39(6):1909-1924. https://doi.org/10.2134/jeq2009.0462 Hou, W.C., Jafvert, C.T., 2009.Photochemical Transformation of Aqueous C-60 Clusters in Sunlight.Environmental Science & Technology, 43(2):362-367. https://doi.org/10.1021/es802465z Huynh, K.A., Chen, K.L., 2011.Aggregation Kinetics of Citrate and Polyvinylpyrrolidone Coated Silver Nanoparticles in Monovalent and Divalent Electrolyte Solutions.Environmental Science & Technology, 45(13):5564-5571. doi: 10.1021/es200157h Huynh, K.A., McCaffery, J.M., Chen, K.L., 2012.Heteroaggregation of Multiwalled Carbon Nanotubes and Hematite Nanoparticles:Rates and Mechanisms.Environmental Science & Technology, 46(11):5912-5920. https://doi.org/10.1021/es2047206 Jiang, C.J., Aiken, G.R., Hsu-Kim, H., 2015.Effects of Natural Organic Matter Properties on the Dissolution Kinetics of Zinc Oxide Nanoparticles.Environmental Science & Technology, 49(19):11476-11484. https://doi.org/10.1021/acs.est.5b02406 Jiang, J.K., Oberdorster, G., Biswas, P., 2009.Characterization of Size, Surface Charge, and Agglomeration State of Nanoparticle Dispersions for Toxicological Studies.Journal of Nanoparticle Research, 11(1):77-89. https://doi.org/10.1007/s11051-008-9446-4 Ju, Y.W., Sun, Y., Wan, Q., et al., 2016.Nanogeology:A Revolutionary Challenge in Geosciences.Bulletin of Mineralogy, Petrology and Geochemistry, 35(1):1-20, 22-23 (in Chinese with English abstract). http://industry.wanfangdata.com.cn/yj/Detail/Periodical?id=Periodical_kwysdqhxtb201601001 Kaegi, R., Voegelin, A., Ort, C., et al., 2013.Fate and Transformation of Silver Nanoparticles in Urban Wastewater Systems.Water Research, 47(12):3866-3877. https://doi.org/10.1016/j.watres.2012.11.060 Kaegi, R., Voegelin, A., Sinnet, B., et al., 2011.Behavior of Metallic Silver Nanoparticles in a Pilot Wastewater Treatment Plant.Environmental Science & Technology, 45(9):3902-3908. https://doi.org/10.1021/es1041892 Keller, A.A., Wang, H.T., Zhou, D.X., et al., 2010.Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices.Environmental Science & Technology, 44(6):1962-1967. https://doi.org/10.1021/es902987d Kim, A.Y., Berg, J.C., 2002.Effect of Polymeric Adlayers on Heteroaggregation Kinetics.Langmuir, 18(9):3418-3422. https://doi.org/10.1021/la015690e Kiser, M.A., Westerhoff, P., Benn, T., et al., 2009.Titanium Nanomaterial Removal and Release from Wastewater Treatment Plants.Environmental Science & Technology, 43(17):6757-6763. https://doi.org/10.1021/es901102n Lebrette, S., Pagnoux, C., Abélard, P., 2004.Stability of Aqueous TiO2 Suspensions:Influence of Ethanol.Journal of Colloid and Interface Science, 280(2):400. doi: 10.1016/j.jcis.2004.07.033 Lee, B.T., Ranville, J.F., 2012.The Effect of Hardness on the Stability of Citrate-Stabilized Gold Nanoparticles and Their Uptake by Daphnia Magma.Journal of Hazardous Materials, 213:434-439. https://doi.org/10.1016/j.jhazmat.2012.02.025 Levard, C., Reinsch, B.C., Michel, F.M., et al., 2011.Sulfidation Processes of PVP-Coated Silver Nanoparticles in Aqueous Solution:Impact on Dissolution Rate.Environmental Science & Technology, 45(12):5260-5266. https://doi.org/10.1021/es2007758 Li, Q.L., Xie, B., Hwang, Y.S., et al., 2009.Kinetics of C-60 Fullerene Dispersion in Water Enhanced by Natural Organic Matter and Sunlight.Environmental Science & Technology, 43(10):3574-3579. https://doi.org/10.1021/es803603x Li, X., Lenhart, J.J., Walker, H.W., 2010.Dissolution-Accompanied Aggregation Kinetics of Silver Nanoparticles.Langmuir, 26(22):16690-16698. https://doi.org/10.1021/la101768n Li, X., Lenhart, J.J., Walker, H.W., 2012a.Aggregation Kinetics and Dissolution of Coated Silver Nanoparticles.Langmuir, 28(2):1095-1104. https://doi.org/10.1021/la202328n Li, Y., Zhang, W., Li, K., et al., 2012b.Oxidative Dissolution of Polymer-Coated CdSe/ZnS Quantum Dots under UV Irradiation:Mechanisms and Kinetics.Environmental Pollution, 164:259-266. https://doi.org/10.1016/j.envpol.2012.01.047 Li, Y., Niu, J.F., Zhang, W., et al., 2014.Influence of Aqueous Media on the ROS-Mediated Toxicity of ZnO Nanoparticles toward Green Fluorescent Protein-Expressing Escherichia Coli under UV-365 Irradiation.Langmuir, 30(10):2852-2862. https://doi.org/10.1021/la5000028 Li, Y., Zhang, W., Niu, J.F., et al., 2013.Surface-Coating-Dependent Dissolution, Aggregation, and Reactive Oxygen Species (ROS) Generation of Silver Nanoparticles under Different Irradiation Conditions.Environmental Science & Technology, 47(18):10293-10301. https://doi.org/10.1021/es400945v Lin, H.F., Liao, S.C., Hung, S.W., 2005.The DC Thermal Plasma Synthesis of ZnO Nanoparticles for Visible-Light Photocatalyst.Journal of Photochemistry and Photobiology A-Chemistry, 174(1):82-87. https://doi.org/10.1016/j.jphotochem.2005.02.015 Liu, J., Aruguete, D.M., Jinschek, J.R., et al., 2008.The Non-Oxidative Dissolution of Galena Nanocrystals:Insights into Mineral Dissolution Rates as a Function of Grain Size, Shape, and Aggregation State.Geochimica et Cosmochimica Acta, 72(24):5984-5996. doi: 10.1016/j.gca.2008.10.010 Liu, J., Aruguete, D.M., Murayama, M., et al., 2009.Influence of Size and Aggregation on the Reactivity of an Environmentally and Industrially Relevant Nanomaterial (PbS).Environmental Science & Technology, 43(21):8178-8183. doi: 10.1021/es902121r%40proofing Liu, J., Pennell, K.G., Hurt, R.H., 2011.Kinetics and Mechanisms of Nanosilver Oxysulfidation.Environmental Science & Technology, 45(17):7345-7353. https://doi.org/10.1021/es201539s Liu, J.F., Legros, S., Ma, G.B., et al., 2012.Influence of Surface Functionalization and Particle Size on the Aggregation Kinetics of Engineered Nanoparticles.Chemosphere, 87(8):918-924. https://doi.org/10.1016/j.chemosphere.2012.01.045 Liu, J.F., Legros, S., von der Kammer, F., et al., 2013.Natural Organic Matter Concentration and Hydrochemistry Influence Aggregation Kinetics of Functionalized Engineered Nanoparticles.Environmental Science & Technology, 47(9):4113-4120. https://doi.org/10.1021/es302447g Liu, J.J., Dai, C., Hu, Y.D., 2018.Aqueous Aggregation Behavior of Citric Acid Coated Magnetite Nanoparticles:Effects of pH, Cations, Anions, and Humic Acid.Environmental Research, 161:49-60. https://doi.org/10.1016/j.envres.2017.10.045 Lohse, S.E., Abadeer, N.S., Zoloty, M., et al., 2017.Nanomaterial Probes in the Environment:Gold Nanoparticle Soil Retention and Environmental Stability as a Function of Surface Chemistry.ACS Sustainable Chemistry & Engineering, 5(12):11451-11458. https://doi.org/10.1021/acssuschemeng.7b02622 Louie, S.M., Gorham, J.M., Tan, J.J., et al., 2017.Ultraviolet Photo-Oxidation of Polyvinylpyrrolidone (PVP) Coatings on Gold Nanoparticles.Environmental Science-Nano, 4(9):1866-1875. https://doi.org/10.1039/c7en00411g Lowry, G.V., Espinasse, B.P., Badireddy, A.R., et al., 2012a.Long-Term Transformation and Fate of Manufactured Ag Nanoparticles in a Simulated Large Scale Freshwater Emergent Wetland.Environmental Science & Technology, 46(13):7027-7036. https://doi.org/10.1021/es204608d Lowry, G.V., Gregory, K.B., Apte, S.C., et al., 2012b.Transformations of Nanomaterials in the Environment.Environmental Science & Technology, 46(13):6893-6899. https://doi.org/10.1021/es300839e Luo, W.H., Hu, W.Y., Xiao, S.F., 2008.Size Effect on the Thermodynamic Properties of Silver Nanoparticles.Journal of Physical Chemistry C, 112(7):2359-2369. https://doi.org/10.1021/jp0770155 Lü, J.T., Zhang, S.Z., Luo, L., et al., 2012.Dissolution and Microstructural Transformation of ZnO Nanoparticles under the Influence of Phosphate.Environmental Science & Technology, 46(13):7215-7221. https://doi.org/10.1021/es301027a Lyon, D.Y., Fortner, J.D., Sayes, C.M., et al., 2005.Bacterial Cell Association and Antimicrobial Activity of a C-60 Water Suspension.Environmental Toxicology and Chemistry, 24(11):2757-2762. https://doi.org/10.1897/04-649r.1 Ma, R., Levard, C., Michel, F.M., et al., 2013.Sulfidation Mechanism for Zinc Oxide Nanoparticles and the Effect of Sulfidation on Their Solubility.Environmental Science & Technology, 47(6):2527-2534. https://doi.org/10.1021/es3035347 Ma, S., Lin, D.H., 2013.The Biophysicochemical Interactions at the Interfaces between Nanoparticles and Aquatic Organisms:Adsorption and Internalization.Environmental Science-Processes & Impacts, 15(1):145-160. https://doi.org/10.1039/c2em30637a Ma, S., Zhou, K.J., Yang, K., et al., 2015.Heteroagglomeration of Oxide Nanoparticles with Algal Cells:Effects of Particle Type, Ionic Strength and pH.Environmental Science & Technology, 49(2):932-939. https://doi.org/10.1021/es504730k Miao, A.J., Zhang, X.Y., Luo, Z.P., et al., 2010.Zinc Oxide Engineered Nanoparticles:Dissolution and Toxicity to Marine Phytoplankton.Environmental Toxicology and Chemistry, 29(12):2814-2822. https://doi.org/10.1002/etc.340 Mikhlin, Y., Romanchenko, A., Likhatski, M., et al., 2011.Understanding the Initial Stages of Precious Metals Precipitation:Nanoscale Metallic and Sulfidic Species of Gold and Silver on Pyrite Surfaces.Ore Geology Reviews, 42(1):47-54. https://doi.org/10.1016/j.oregeorev.2011.03.005 Misawa, M., Takahashi, J., 2011.Generation of Reactive Oxygen Species Induced by Gold Nanoparticles under X-Ray and UV Irradiations.Nanomedicine-Nanotechnology Biology and Medicine, 7(5):604-614. https://doi.org/10.1016/j.nano.2011.01.014 Misra, S.K., Dybowska, A., Berhanu, D., et al., 2012a.Isotopically Modified Nanoparticles for Enhanced Detection in Bioaccumulation Studies.Environmental Science & Technology, 46(2):1216-1222. https://doi.org/10.1021/es2039757 Misra, S.K., Dybowska, A., Berhanu, D., et al., 2012b.The Complexity of Nanoparticle Dissolution and Its Importance in Nanotoxicological Studies.Science of the Total Environment, 438:225-232. https://doi.org/10.1016/j.scitotenv.2012.08.066 Misra, S.K., Nuseibeh, S., Dybowska, A., et al., 2014.Comparative Study Using Spheres, Rods and Spindle-Shaped Nanoplatelets on Dispersion Stability, Dissolution and Toxicity of Cuo Nanomaterials.Nanotoxicology, 8(4):422-432. https://doi.org/10.3109/17435390.2013.796017 Mulvihill, M.J., Habas, S.E., Jen-La Plante, H., et al., 2010.Influence of Size, Shape, and Surface Coating on the Stability of Aqueous Suspensions of CdSe Nanoparticles.Chemistry of Materials, 22(18):5251-5257. https://doi.org/10.1021/cm101262s Muntean, J.L., Cline, J.S., Simon, A.C., et al., 2011.Magmatic-Hydrothermal Origin of Nevada's Carlin-Type Gold Deposits.Nature Geoscience, 4(2):122-127. doi: 10.1038/ngeo1064 Navrotsky, A., Mazeina, L., Majzlan, J., 2008.Size-Driven Structural and Thermodynamic Complexity in Iron Oxides.Science, 319(5870):1635-1638. https://doi.org/10.1126/science.1148614 Novikov, A.P., Kalmykov, S.N., Utsunomiya, S., et al., 2006.Colloid Transport of Plutonium in the Far-Field of the Mayak Production Association, Russia.Science, 314(5799):638-641. https://doi.org/10.1126/science.1131307 Odzak, N., Kistler, D., Behra, R., et al., 2014.Dissolution of Metal and Metal Oxide Nanoparticles in Aqueous Media.Environmental Pollution, 191:132-138. https://doi.org/10.1016/j.envpol.2014.04.010 Oo, M.K.K., Yang, Y.M., Hu, Y., et al., 2012.Gold Nanoparticle-Enhanced and Size-Dependent Generation of Reactive Oxygen Species from Protoporphyrin Ⅸ.ACS Nano, 6(3):1939-1947. https://doi.org/10.1021/nn300327c Peng, C., Shen, C.S., Zheng, S.Y., et al., 2017.Transformation of CuO Nanoparticles in the Aquatic Environment:Influence of pH, Electrolytes and Natural Organic Matter.Nanomaterials, 7(10). https://doi.org/10.3390/nano7100326 Phenrat, T., Saleh, N., Sirk, K., et al., 2007.Aggregation and Sedimentation of Aqueous Nanoscale Zero Valent Iron Dispersions.Environmental Science & Technology, 41(1):284-290. https://doi.org/10.1021/es061349a Phenrat, T., Saleh, N., Sirk, K., et al., 2008.Stabilization of Aqueous Nanoscale Zero Valent Iron Dispersions by Anionic Polyelectrolytes:Adsorbed Anionic Polyelectrolyte Layer Properties and Their Effect on Aggregation and Sedimentation.Journal of Nanoparticle Research, 10(5):795-814. https://doi.org/10.1007/s11051-007-9315-6 Putnis, C.V., Ruiz-Agudo, E., 2013.The Mineral-Water Interface:Where Minerals React with the Environment.Elements, 9(3):177-182. https://doi.org/10.2113/gselements.9.3.177 Quik, J.T.K., Stuart, M.C., Wouterse, M., et al., 2012.Natural Colloids are the Dominant Factor in the Sedimentation of Nanoparticles.Environmental Toxicology and Chemistry, 31(5):1019-1022. https://doi.org/10.1002/etc.1783 Quik, J.T.K., Vonk, J.A., Hansen, S.F., et al., 2011.How to Assess Exposure of Aquatic Organisms to Manufactured Nanoparticles? Environment International, 37(6):1068-1077. https://doi.org/10.1016/j.envint.2011.01.015 Rathnayake, S., Unrine, J.M., Judy, J., et al., 2014.Multitechnique Investigation of the pH Dependence of Phosphate Induced Transformations of ZnO Nanoparticles.Environmental Science & Technology, 48(9):4757-4764. https://doi.org/10.1021/es404544w Rebodos, R.L., Vikesland, P.J., 2010.Effects of Oxidation on the Magnetization of Nanoparticulate Magnetite.Langmuir, 26(22):16745-16753. https://doi.org/10.1021/la102461z Reich, M., Hough, R.M., Deditius, A., et al., 2011.Nanogeoscience in Ore Systems Research:Principles, Methods, and Applications Introduction and Preface to the Special Issue Preface.Ore Geology Reviews, 42(1):1-5. https://doi.org/10.1016/j.oregeorev.2011.06.007 Reich, M., Kesler, S.E., Utsunomiya, S., et al., 2005.Solubility of Gold in Arsenian Pyrite.Geochimica et Cosmochimica Acta, 69(11):2781-2796. doi: 10.1016/j.gca.2005.01.011 Reinsch, B.C., Levard, C., Li, Z., et al., 2012.Sulfidation of Silver Nanoparticles Decreases Escherichia Coli Growth Inhibition.Environmental Science & Technology, 46(13):6992-7000. https://doi.org/10.1021/es203732x Rhiem, S., Riding, M.J., Baumgartner, W., et al., 2015.Interactions of Multiwalled Carbon Nanotubes with Algal Cells:Quantification of Association, Visualization of Uptake, and Measurement of Alterations in the Composition of Cells.Environmental Pollution, 196:431-439. https://doi.org/10.1016/j.envpol.2014.11.011 Romanchenko, A., Mikhlin, Y.L., Makhova, L., 2007.Investigation of Gold Nanoparticles Immobilized on the Surface of Pyrite by Scanning Probe Microscopy, Scanning Tunneling Spectroscopy, and X-Ray Photoelectron Spectroscopy.Glass Physics and Chemistry, 33(4):417-421. doi: 10.1134/S1087659607040177 Saunders, J.A., 1990.Colloidal Transport of Gold and Silica in Epithermal Precious-Metal Systems-Evidence from the Sleeper Deposit, Nevada.Geology, 18(8):757-760.https://doi.org/10.1130/0091-7613(1990)018 <0757:ctogas>2.3.co; 2 doi: 10.1130/0091-7613(1990)018<0757:ctogas>2.3.co;2 Saunders, J.A., Burke, M., 2017.Formation and Aggregation of Gold (Electrum) Nanoparticles in Epithermal Ores.Minerals, 7(9):163. https://doi.org/10.3390/min7090163 Siy, J.T., Bartl, M.H., 2010.Insights into Reversible Dissolution of Colloidal CdSe Nanocrystal Quantum Dots.Chemistry of Materials, 22(21):5973-5982. https://doi.org/10.1021/cm102156v Stankus, D.P., Lohse, S.E., Hutchison, J.E., et al., 2011.Interactions between Natural Organic Matter and Gold Nanoparticles Stabilized with Different Organic Capping Agents.Environmental Science & Technology, 45(8):3238-3244. https://doi.org/10.1021/es102603p Stipp, S.L.S., Hansen, M., Kristensen, R., et al., 2002.Behaviour of Fe-Oxides Relevant to Contaminant Uptake in the Environment.Chemical Geology, 190(1-4):321-337. https://doi.org/10.1016/s0009-2541(02)00123-7 Stumm, W., 1992. Chemistry of The Solid-Water Interface. Wiley-Interscience, New York. Stumm, W., 1996. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. Wiley, New York. Stumm, W., Morgan, J. J., 1981. Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters. Wiley & Sons, New York. Verma, A., Stellacci, F., 2010.Effect of Surface Properties on Nanoparticle-Cell Interactions.Small, 6(1):12-21. https://doi.org/10.1002/smll.200901158 Verwey, E. J. W., Owerbeek, J. T. G., 1948. The Theory of the Stability of Liophobic Colloids: The Interaction of Sol Particles Having an Electric Double Layer. Elsevier, Amsterdam. Wagner, S., Gondikas, A., Neubauer, E., et al., 2014.Spot the Difference:Engineered and Natural Nanoparticles in the Environment-Release, Behavior, and Fate.Angewandte Chemie-International Edition, 53(46):12398-12419. https://doi.org/10.1002/anie.201405050 Wan, Q., Qin, Z.H., Ju, Y.W., et al., 2016.Nanogeochemistry:Origin, Recent Advances and Future Directions.Bulletin of Mineralogy, Petrology and Geochemistry, 35(1):21-27 (in Chinese with English abstract). Wang, H.T., Adeleye, A.S., Huang, Y.X., et al., 2015a.Heteroaggregation of Nanoparticles with Biocolloids and Geocolloids.Advances in Colloid and Interface Science, 226:24-36. https://doi.org/10.1016/j.cis.2015.07.002 Wang, P., Du, M., Zhu, H., et al., 2015b.Structure Regulation of Silica Nanotubes and Their Adsorption Behaviors for Heavy Metal Ions:pH Effect, Kinetics, Isotherms and Mechanism.Journal of Hazardous Materials, 286:533-544. https://doi.org/10.1016/j.jhazmat.2014.12.034 Wang, Y.F., 2014.Nanogeochemistry:Nanostructures, Emergent Properties and Their Control on Geochemical Reactions and Mass Transfers.Chemical Geology, 378:1-23. https://doi.org/10.1016/j.chemgeo.2014.04.007 Wang, Z.Y., Li, J., Zhao, J., et al., 2011.Toxicity and Internalization of CuO Nanoparticles to Prokaryotic Alga Microcystis Aeruginosa as Affected by Dissolved Organic Matter.Environmental Science & Technology, 45(14):6032-6040. https://doi.org/10.1021/es2010573 Wieder, M.E., Hone, D.C., Cook, M.J., et al., 2006.Intracellular Photodynamic Therapy with Photosensitizer-Nanoparticle Conjugates:Cancer Therapy Using a 'Trojan Horse'.Photochemical & Photobiological Sciences, 5(8):727-734. https://doi.org/10.1039/b602830f Yang, K., Lin, D., Xing, B., 2009.Interactions of Humic Acid with Nanosized Inorganic Oxides.Langmuir, 25(6):3571-3576. https://doi.org/10.1021/la803701b Zhang, B.R., Fu, J.M., 2005.Advances in Geochemistry.Chemical Industry Press, Beijing, 322-325 (in Chinese). Zhang, H., Chen, B., Ren, Y., et al., 2010a.Response of Nanoparticle Structure to Different Types of Surface Environments:Wide-Angle X-Ray Scattering and Molecular Dynamics Simulations.Physical Review B, 81(12). https://doi.org/10.1103/PhysRevB.81.125444 Zhang, H.Z., Chen, B., Banfield, J.F., 2010b.Particle Size and pH Effects on Nanoparticle Dissolution.Journal of Physical Chemistry C, 114(35):14876-14884. https://doi.org/10.1021/jp1060842 Zhang, W.C., Xiao, B.D., Fang, T., 2018.Chemical Transformation of Silver Nanoparticles in Aquatic Environments:Mechanism, Morphology and Toxicity.Chemosphere, 191:324-334. https://doi.org/10.1016/j.chemosphere.2017.10.016 Zhou, D.X., Abdel-Fattah, A.I., Keller, A.A., 2012.Clay Particles Destabilize Engineered Nanoparticles in Aqueous Environments.Environmental Science & Technology, 46(14):7520-7526. https://doi.org/10.1021/es3004427 陈天虎, 陈骏, 季峻峰, 等, 2005.洛川黄土纳米尺度观察:纳米棒状方解石.地质论评, 51(6):713-718, 741-742. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dzlp200506014 成东, 廖鹏, 袁松虎, 2016.FeS胶体对三价铁吸附态As(Ⅴ)的解吸作用.地球科学, 41(2):325-330. http://www.earth-science.net/WebPage/Article.aspx?id=3249 何宏平, 郭龙皋, 谢先德, 等, 1999.蒙脱石等粘土矿物对重金属离子吸附选择性的实验研究.矿物学报, 19(2):231-235. http://www.cnki.com.cn/Article/CJFDTOTAL-KWXB199902015.htm 何宏平, 郭九皋, 朱建喜, 等, 2001.蒙脱石、高岭石、伊利石对重金属离子吸附容量的实验研究.岩石矿物学杂志, 20(4):573-578. https://www.wenkuxiazai.com/doc/a9d63cfd52ea551811a687be.html 琚宜文, 孙岩, 万泉, 等, 2016.纳米地质学:地学领域革命性挑战.矿物岩石地球化学通报, 35(1):1-20, 22-23. http://www.cqvip.com/QK/84215X/201601/668146269.html 万泉, 覃宗华, 琚宜文, 等, 2016.纳米地球化学刍析:起源、研究进展和发展方向.矿物岩石地球化学通报, 35(1):21-27. http://industry.wanfangdata.com.cn/yj/Detail/Periodical?id=Periodical_kwysdqhxtb201601002 张本仁, 傅家谟, 2005.地球化学进展.北京:化学工业出版社, 322-325. -
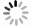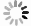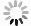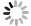








 下载:
下载: