Raman Spectroscopy of Ion Exchange in Interlayer of Triclinic Birnessite
-
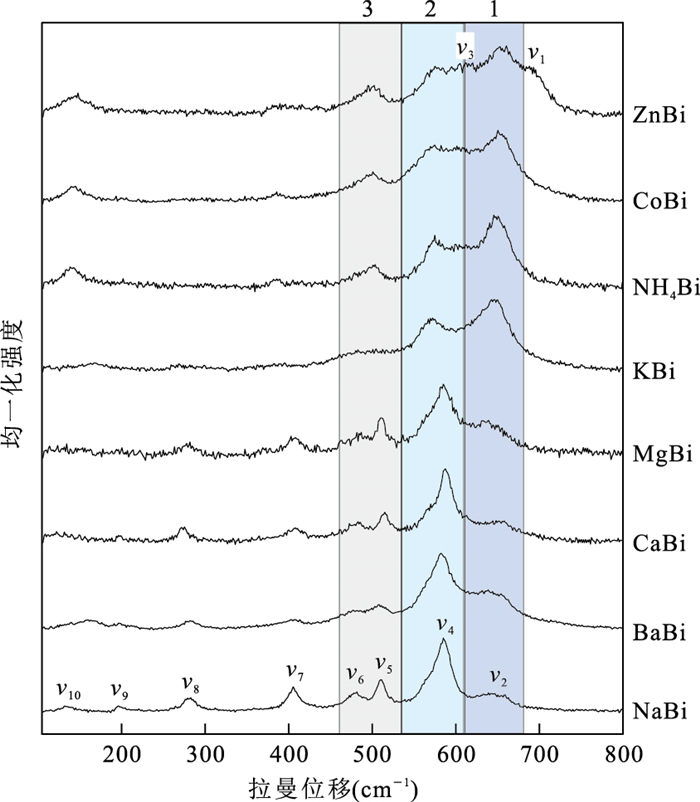
摘要: 水钠锰矿为自然界中常见的锰氧化物矿物,其离子交换作用及结构转变理解尚不深刻,矿物表征手段较为局限.为探究水钠锰矿的离子交换特性以及结构转变在拉曼光谱上的反映,利用MnSO4和NaOH合成了三斜晶系的Na型水钠锰矿,进行了NH4+、K+、Mg2+、Ca2+、Ba2+、Co2+、Zn2+的离子交换实验,使用ICP-OES、XRD、拉曼光谱等手段对离子交换水钠锰矿进行表征.拉曼光谱分析表明,570~585 cm-1与640~655 cm-1两个锰氧八面体伸缩振动模式的相对强度及570~585 cm-1附近拉曼峰峰位指示水钠锰矿的结构对称型;570~585 cm-1拉曼峰强度大、振动频率高指示三斜对称型.280 cm-1与500 cm-1附近的拉曼峰是层间离子种类的识别标志.水钠锰矿层间若为Na+、K+、Mg2+、Ca2+、Ba2+等碱金属、碱土金属离子,则在280 cm-1附近存在1个峰值,500 cm-1存在2个分立的峰值;其他种类的层间离子仅500 cm-1处有1个孤峰,指示层间离子排列无序.Abstract: Birnessite is a group of manganese oxide minerals widely found in nature. However, its ion exchange behavior and structural transformation have not been fully understood, and the characterization techniques are limited. To study the ion exchange behavior of birnessite and the reflection of structural transformation in Raman spectroscopy, triclinic Na-birnessite was synthesized using MnSO4 and NaOH, and ion exchange experiments on NH4+, K+, Mg2+, Ca2+, Ba2+, Co2+, and Zn2+ were carried out. Ion exchange birnessite samples were characterized using ICP-OES, XRD, and Raman spectroscopy. Raman study shows the relative strength of two stretching vibration modes in[MnO6] octahedra around 570-585 cm-1 and 640-655 cm-1 and the band location of the mode around 570-585 cm-1 are indicators of the symmetry of birnessite. High strength and frequency of the mode around 570-585 cm-1 are signs of triclinic symmetry. Raman bands around 280 cm-1 and 500 cm-1 are indicators of interlayer cations. If alkalis and alkaline-earth metals (i.e., Na+, K+, Mg2+, Ca2+, Ba2+, etc.) are in the interlayer of birnessite, a band around 280 cm-1 and two separate bands around 500 cm-1 will appear; whereas other interlayer cations only give rise to one single band at 500 cm-1, indicating disorder in the interlayer.
-
Key words:
- birnessite /
- ion exchange /
- Raman spectroscopy /
- structural symmetry /
- interlayer distance /
- mineralogy
-
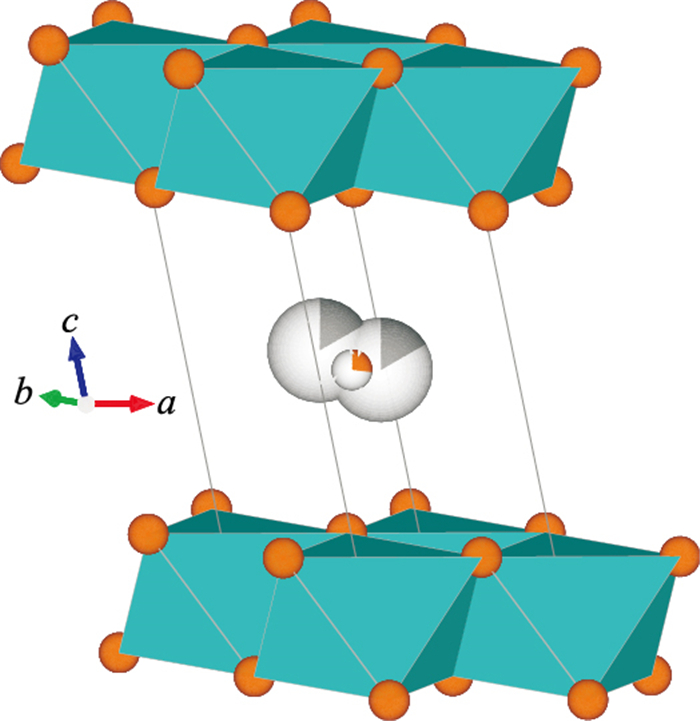
图 1 三斜水钠锰矿的晶体结构
据Lanson et al.(2002)确定的晶体结构重绘.三斜水钠锰矿中层内Mn为+3或+4价,结构八面体(图中蓝色八面体)配体为氧原子(图中橙色原子);层间含大半径阳离子(如Na+等,图中以灰色原子表示)与水分子(图中以氧原子表示)
Fig. 1. Crystal structure of triclinic birnessite
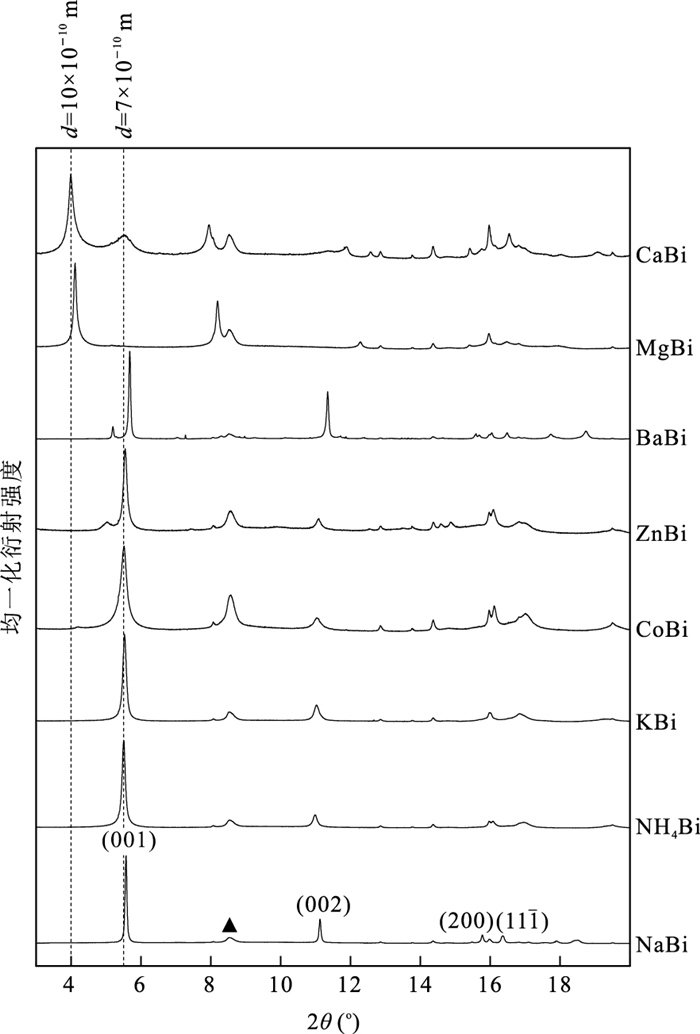
图 3 15.5°~33°范围内NaBi、KBi、NH4Bi、CoBi、ZnBi的XRD图谱
a.15.5°~17.5°范围内5种水钠锰矿的衍射图,三斜水钠锰矿的(200)、(111)衍射峰在NaBi中标出;KBi、NH4Bi、CoBi、ZnBi中指示三斜对称型的衍射峰消失,后3种水钠锰矿中还可见六方水钠锰矿的(100)和(101)衍射峰.b.18°~33°范围内5种水钠锰矿的衍射图,在KBi、NH4Bi、CoBi和ZnBi中均出现了d值约为2.03 ×10-10 m、1.71 ×10-10 m左右的指示六方对称型的(102)、(103)特征衍射峰,并且观察到了六方水钠锰矿的(104)、(110)、(111)与(112)衍射峰.图中三角形指示水黑锰矿杂质的衍射峰
Fig. 3. XRD patterns of NaBi, KBi, NH4Bi, CoBi and ZnBi in the range of 15.5°-33°
表 1 不同种类水钠锰矿阳离子半径、离子水合能、化学组成及层间距
Table 1. Ionic radii, hydration energies, chemical compositions, and interlayer distances of different kinds of birnessite
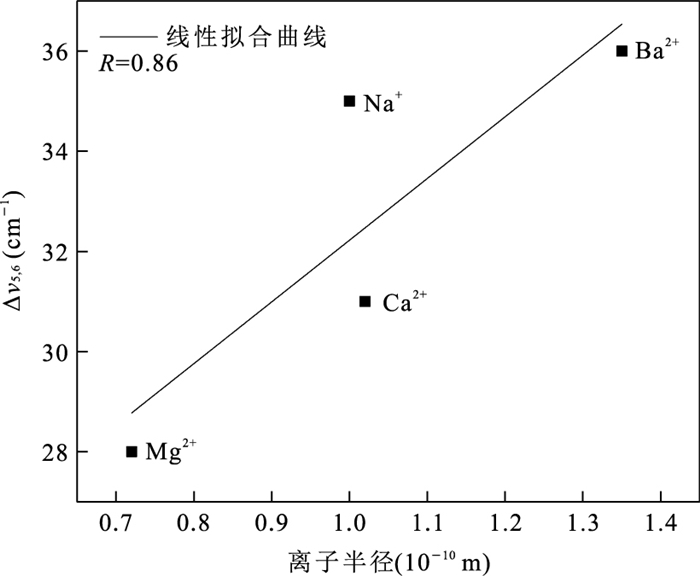
阳离子 有效离子半径(10-10 m)a 离子水合能(kcal/g)b Na/Mn摩尔比(%) 交换离子/Mn摩尔比(%) 总阳离子含量(%)d 层间距(10-10 m) Na+ 1.02 98.2 19.77 - 19.77 7.088 NH4+ 1.40b - 0.19 -c 7.162 K+ 1.38 80.6 0.19 12.32 12.51 7.136 Mg2+ 0.72 455.5 0.25 12.81 25.87 9.590 Ca2+ 1.00 380.8 0.20 17.29 34.78 7.126、9.895 Ba2+ 1.35 315.1 1.89 13.33 28.55 6.953 Co2+ 0.65 479.5 0.95 10.94 22.83~33.77e 7.149、9.423 Zn2+ 0.74 484.6 2.58 34.74 72.06 7.125 注:a.所有离子半径均选择六配位状态的,过渡金属离子半径选择低自旋条件下的(Shannon,1976);b.数据引用自Rosseinsky(1965);c.该测试手段无法测定NH4+含量;d.按照阳离子含量×阳离子电荷计算;e.CoBi中Co可能为+2或+3两种价态,按照两端元情况计算得到含量范围. 表 2 8种离子交换水钠锰矿样品拉曼峰位统计
Table 2. Summary table of Raman bands in eight kinds of ion exchange birnessite
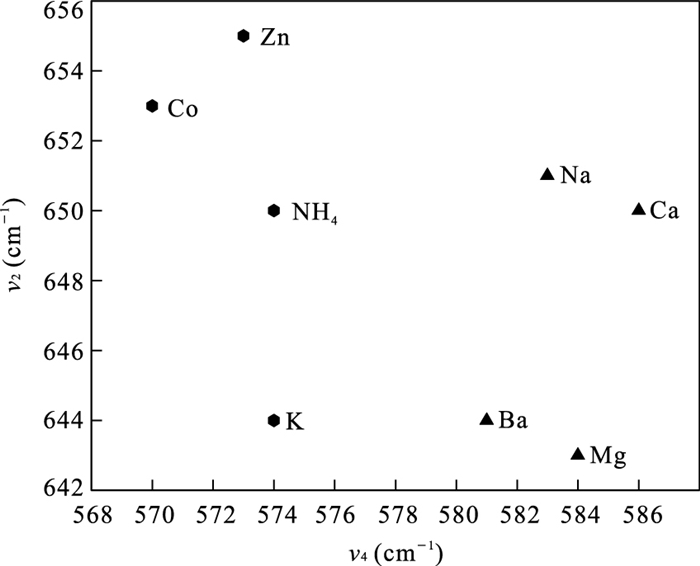
模式 NaBi MgBi NH4Bi KBi CaBi CoBi ZnBi BaBi v1 692(sh) v2 651(m) 643(m) 650(s) 645(s) 650(m) 653(s) 655(s) 644(m) v3 608(vw) 609(vw) 612(w) v4 583(s) 584(s) 577(s) 575(s) 586(s) 570(s) 573(s) 581(s) v5 511(m) 511(m) 498(m) 509(w) 515(m) 496(m) 497(m) 508(m) v6 480(sh) 483(sh) 484(sh) 480(sh) 472(sh) v7 406(m) 407(m) 387(w) 393(vw) 407(m) 386(w) 403(w) 397(w) v8 283(w) 278(m) 280(w) 278(m) 282(w) v9 199(w) 198(vw) 201(vw) 198(w) 199(vw) 214(vw) 204(vw) v10 150(vw) 140(vw) 142(m) 165(w) 140(vw) 142(m) 145(m) 163(w) 注:振动频率单位均为cm-1.vs.很强;s.强;m.中等;w.弱;vw.很弱;sh.肩峰.在NH4Bi、CoBi、ZnBi中,v5与v6振动简并为一个拉曼峰. 表 3 离子交换水钠锰矿[MnO6]两种伸缩振动模式强度之比
Table 3. Relative strength of two [MnO6] stretching vibration modes in different kinds of ion exchange birnessite
样品 NaBi MgBi CaBi BaBi NH4Bi KBi CoBi ZnBi v4/v2强度 4.32±1.37 2.97±0.76 2.70±0.35 1.39±0.42 0.43±0.03 0.66±0.12 0.56±0.01 0.77±0.15 对称型 三斜 三斜 三斜 三斜 六方 六方 六方 六方 注:每个样品按照两个峰强的积分面积进行比较,取3个及以上数据进行统计,正负号之后为样本标准差. -
Al-Attar, L., Dyer, A., 2007.Ion Exchange in Birnessite.Land Contamination & Reclamation, 15(4):427-436. https://doi.org/10.2462/09670513.878 Bargar, J.R., Fuller, C.C., Marcus, M.A., et al., 2009.Structural Characterization of Terrestrial Microbial Mn Oxides from Pinal Creek, AZ.Geochimica et Cosmochimica Acta, 73(4):889-910. https://doi.org/10.1016/j.gca.2008.10.036 Burns, R. G., Burns, V. M., 1979. Manganese Oxides. In: Burns, R. G., ed., Marine Minerals. Mineral Society of America, Cambridge, 1-46. Deibert, B.J., Zhang, J., Smith, P.F., et al., 2015.Surface and Structural Investigation of a MnOx Birnessite-Type Water Oxidation Catalyst Formed under Photocatalytic Conditions.Chemistry, 21(40):14218-14228. https://doi.org/10.1002/chem.201501930 Drits, V.A., Silvester, E., Gorshkov, A., et al., 1997.Structure of Synthetic Monoclinic Na-Rich Birnessite and Hexagonal Birnessite:I.Results from X-Ray Diffraction and Selected-Area Electron Diffraction.American Mineralogist, 82 (9-10):962-978. https://doi.org/10.2138/am-1997-9-1012 Fan, C., Wang, L., Fan, X., et al., 2015.The Mineralogical Characterization of Argentian Cryptomelane from Xiangguang Mn-Ag Deposit, North China.Journal of Mineralogical and Petrological Sciences, 110(5):214-223. https://doi.org/10.2465/jmps.150119 Feng, Q., Kanoh, H., Ooi, K., 1999.Manganese Oxide Porous Crystals.Journal of Materials Chemistry, 9(2):319-333. https://doi.org/10.1039/A805369C Feng, X.H., Tan, W.F., Liu, F., et al., 2003.Synthesis of Birnessite in Alkali Media and Its Transformation to Todorokite.Bulletin of Mineralogy, Petrology and Geochemistry, 22(2):184-187 (in Chinese with English abstract). Gaillot, A.C., Drits, V.A., Manceau, A., et al., 2006.Structure of the Synthetic K-Rich Phyllomanganate Birnessite Obtained by High-Temperature Decomposition of KMnO4.Substructures of K-Rich Birnessite from 1 000℃ Experiment.Microporous and Mesoporous Materials, 98(1-3):267-282. https://doi.org/10.1016/j.micromeso.2006.09.010 Gaillot, A.C., Flot, D., Drits, V.A., et al., 2003.Structure of Synthetic K-Rich Birnessite Obtained by High-Temperature Decomposition of KMnO4.I.Two-Layer Polytype from 800℃ Experiment.Chemistry of Materials, 15(25):4666-4678. https://doi.org/10.1021/cm021733g Gao, T., Fjellvåg, H., Norby, P., 2009.A Comparison Study on Raman Scattering Properties of α-and β-MnO2.Analytica Chimica Acta, 648(2):235-239. https://doi.org/10.1016/j.aca.2009.06.059 Gao, T., Glerup, M., Krumeich, F., et al., 2008.Microstructures and Spectroscopic Properties of Cryptomelane-Type Manganese Dioxide Nanofibers.Journal of Physical Chemistry C, 112(34):13134-13140. https://doi.org/10.1021/jp804924f Giovanoli, R., Stähli, E., Feitknecht, W., 1970.Vber Oxidhydroxide des Vierwertigen Mangans Mit Schichtengitter.1.Mitteilung.Natriummangan(Ⅱ, Ⅲ) Manganat(Ⅳ).Helvetica Chimica Acta, 53(2):209-220. https://doi.org/10.1002/hlca.19700530203 Golden, D.C., 1986.Ion Exchange, Thermal Transformations, and Oxidizing Properties of Birnessite.Clays and Clay Minerals, 34(5):511-520. https://doi.org/10.1346/CCMN.1986.0340503 Hsu, Y.K., Chen, Y.C., Lin, Y.G., et al., 2011.Reversible Phase Transformation of MnO2 Nanosheets in an Electrochemical Capacitor Investigated by In-Situ Raman Spectroscopy.Chemical Communications, 47(4):1252-1254. https://doi.org/10.1039/C0CC03902K Johnson, E.A., Post, J.E., 2006.Water in the Interlayer Region of Birnessite:Importance in Cation Exchange and Structural Stability.American Mineralogist, 91(4):609-618. https://doi.org/10.2138/am.2006.2090 Jones, L.H.P., Milne, A.A., 1956.Birnessite, a New Manganese Oxide Mineral from Aberdeenshire, Scotland.Mineralogical Magazine, 31(235):283-288. http://minersoc.org/pages/Archive-MM/Volume_31/31-235-283.htm Julien, C., 2000. Local Environment in 4-Volt Cathode Materials for Li-Ion Batteries. In: Julien, C., Stoynov, Z., eds., Materials for Lithium-Ion Batteries. Springer Netherlands, Dordrecht, 309-326. https://doi.org/10.1007/978-94-011-4333-2_13 Julien, C., Massot, M., Baddour-Hadjean, R., et al., 2003.Raman Spectra of Birnessite Manganese Dioxides.Solid State Ionics, 159(3-4):345-356. https://doi.org/10.1016/S0167-2738(03)00035-3 Julien, C., Massot, M., Rangan, S., et al., 2002.Study of Structural Defects in γ-MnO2 by Raman Spectroscopy.Journal of Raman Spectroscopy, 33(4):223-228. https://doi.org/10.1002/jrs.838 Julien, C.M., Massot, M., 2003.Lattice Vibrations of Materials for Lithium Rechargeable Batteries Ⅲ.Lithium Manganese Oxides.Materials Science and Engineering:B, 100(1):69-78. https://doi.org/10.1016/S0921-5107(03)00077-1 Julien, C.M., Massot, M., Poinsignon, C., 2004.Lattice Vibrations of Manganese Oxides:Part Ⅰ.Periodic Structures.Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 60(3):689-700. https://doi.org/10.1016/S1386-1425(03)00279-8 Kang, L., Zhang, M., Liu, Z.H., et al., 2007.IR Spectra of Manganese Oxides with Either Layered or Tunnel Structures.Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 67(3-4):864-869. https://doi.org/10.1016/j.saa.2006.09.001 Kim, H.S., Stair, P.C., 2004.Bacterially Produced Manganese Oxide and Todorokite:UV Raman Spectroscopic Comparison.Journal of Physical Chemistry B, 108(44):17019-17026. https://doi.org/10.1021/jp048810a Kuma, K., 1994.Crystal Structures of Synthetic 7 Å and 10 Å Manganates Substituted by Mono-and Divalent Cations.Mineralogical Magazine, 58(392):425-447. https://doi.org/10.1180/minmag.1994.058.392.08 Kwon, K.D., Refson, K., Sposito, G., 2009.Zinc Surface Complexes on Birnessite:A Density Functional Theory Study.Geochimica et Cosmochimica Acta, 73(5):1273-1284. https://doi.org/10.1016/j.gca.2008.11.033 Kwon, K.D., Refson, K., Sposito, G., 2013.Understanding the Trends in Transition Metal Sorption by Vacancy Sites in Birnessite.Geochimica et Cosmochimica Acta, 101:222-232. https://doi.org/10.1016/j.gca.2012.08.038 Lanson, B., Drits, V.A., Feng, Q., et al., 2002.Structure of Synthetic Na-Birnessite:Evidence for a Triclinic One-Layer Unit Cell.American Mineralogist, 87 (11-12):1662-1671. https://doi.org/10.2138/am-2002-11-1215 Lanson, B., Drits, V.A., Silvester, E., et al., 2000.Structure of H-Exchanged Hexagonal Birnessite and Its Mechanism of Formation from Na-Rich Monoclinic Buserite at Low pH.American Mineralogist, 85(5-6):826-838. https://doi.org/10.2138/am-2000-5-625 Le Goff, P., Baffier, N., Bach, S., 1996.Synthesis, Ion Exchange and Electrochemical Properties of Lamellar Phyllomanganates of the Birnessite Group.Materials Research Bulletin, 31(1):63-75. https://doi.org/10.1016/0025-5408(95)00170-0 Le Goff, P., Baffier, N., Bach, S., et al., 1993.Structural and Electrochemical Characteristics of a Lamellar Sodium Manganese Oxide Synthesized via a Sol-Gel Process.Solid State Ionics, 61(4):309-315. https://doi.org/10.1016/0167-2738(93)90397-L Ling, F.T., Heaney, P.J., Post, J.E., et al., 2015.Transformations from Triclinic to Hexagonal Birnessite at Circumneutral pH Induced through pH Control by Common Biological Buffers.Chemical Geology, 416:1-10. https://doi.org/10.1016/j.chemgeo.2015.10.007 Ling, F.T., Post, J.E., Heaney, P.J., et al., 2017.Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of Triclinic and Hexagonal Birnessites.Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 178:32-46. https://doi.org/10.1016/j.saa.2017.01.032 Lopano, C.L., Heaney, P.J., Bandstra, J.Z., et al., 2011.Kinetic Analysis of Cation Exchange in Birnessite Using Time-Resolved Synchrotron X-Ray Diffraction.Geochimica et Cosmochimica Acta, 75(14):3973-3981. https://doi.org/10.1016/j.gca.2011.04.021 Lopano, C.L., Heaney, P.O., Post, J.E., 2007.Time-Resolved Structural Analysis of K-and Ba-Exchange Reactions with Synthetic Na-Birnessite Using Synchrotron X-Ray Diffraction.American Minerologist, 92(2-3):380-387. https://doi.org/10.2138/am.2007.2242 Lopano, C.L., Heaney, P.J., Post, J.E., 2009.Cs-Exchange in Birnessite:Reaction Mechanisms Inferred from Time-Resolved X-Ray Diffraction and Transmission Electron Microscopy.American Mineralogist, 94(5-6):816-826. https://doi.org/10.2138/am.2009.3068 Manceau, A., Drits, V.A., Silvester, E., et al., 1997.Structural Mechanism of Co2+ Oxidation by the Phyllomanganate Buserite.American Mineralogist, 82(11-12):1150-1175. https://doi.org/10.2138/am-1997-11-1213 Manceau, A., Lanson, B., Drits, V.A., et al., 2002.Structure of Heavy Metal Sorbed Birnessite.Part Ⅲ:Results from Powder and Polarized Extended X-Ray Absorption Fine Structure Spectroscopy.Geochimica et Cosmochimica Acta, 66(15):2639-2663. https://doi.org/10.1016/S0016-7037(02)00869-4 Matsui, H., Ju, J., Odaira, T., et al., 2009.Two-Dimensionally Confined Water in between MnO2 Layers of Na-Birnessite.Journal of the Physical Society of Japan, 78(7):1-6. https://doi.org/10.1143/JPSJ.78.074801 McKenzie, R.M., 1971.The Synthesis of Birnessite, Cryptomelane, and Some Other Oxides and Hydroxides of Manganese.Mineralogical Magazine, 38(296):493-502. https://doi.org/10.1180/minmag.1971.038.296.12 McKeown, D.A., Post, J.E., 2001.Characterization of Manganese Oxide Mineralogy in Rock Varnish and Dendrites Using X-Ray Absorption Spectroscopy.American Mineralogist, 86(5-6):701-713. https://doi.org/10.2138/am-2001-5-611 Peña, J., Bargar, J.R., Sposito, G., 2015.Copper Sorption by the Edge Surfaces of Synthetic Birnessite Nanoparticles.Chemical Geology, 396:196-207. https://doi.org/10.1016/j.chemgeo.2014.12.021 Pitarch, À., Ruiz, J.F., de Vallejuelo, S.F.O., et al., 2014.In Situ Characterization by Raman and X-Ray Fluorescence Spectroscopy of Post-Paleolithic Blackish Pictographs Exposed to the Open Air in Los Chaparros Shelter (Albalate del Arzobispo, Teruel, Spain).Analytical Methods, 6(17):6641-6650. https://doi.org/10.1039/C4AY00539B Post, J.E., Appleman, D.E., 1988.Chalcophanite, ZnMn3O7·3H2O:New Crystal-Structure Determinations.American Mineralogist, 73(11-12):1401-1404. http://www.researchgate.net/publication/279891943_Chalcophanite_ZnMn3O73H2O_new_crystal-structure_determinations Post, J.E., Heaney, P.J., Hanson, J., 2002.Rietveld Refinement of a Triclinic Structure for Synthetic Na-Birnessite Using Synchrotron Powder Diffraction Data.Powder Diffraction, 17(3):218-221. https://doi.org/10.1154/1.1498279 Post, J.E., Veblen, D.R., 1990.Crystal Structure Determinations of Synthetic Sodium, Magnesium, and Potassium Birnessite Using TEM and the Rietveld Method.American Mineralogist, 75(5-6):477-489. https://www.researchgate.net/publication/279891700_Crystal_structure_determinations_of_synthetic_sodium_magnesium_and_potassium_birnessite_using_TEM_and_the_Rietveld_method Potter, R.M., Rossman, G.R., 1979.The Tetravalent Manganese Oxides:Identification, Hydration, and Structural Relationships by Infrared Spectroscopy.American Mineralogist, 64 (11-12):1199-1218. https://core.ac.uk/display/12816742 Rosseinsky, D.R., 1965.Electrode Potentials and Hydration Energies.Theories and Correlations, Chemical Reviews, 65(4):467-490. https://doi.org/10.1021/cr60236a004 Rousseau, D.L., Bauman, R.P., Porto, S.P.S., 1981.Normal Mode Determination in Crystals.Journal of Raman Spectroscopy, 10(1):253-290. https://doi.org/10.1002/jrs.1250100152 Shannon, R.D., 1976.Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides.Acta Crystallographica Section A, 32(5):751-767. https://doi.org/10.1107/S0567739476001551 Silvester, E., Manceau, A., Drits, V.A., 1997.Structure of Synthetic Monoclinic Na-Rich Birnessite and Hexagonal Birnessite:Ⅱ.Results from Chemical Studies and EXAFS Spectroscopy.American Mineralogist, 82(9-10):962-978. https://doi.org/10.2138/am-1997-9-1013 Webb, S.M., Dick, G.J., Bargar, J.R., et al., 2005a.Evidence for the Presence of Mn(Ⅲ) Intermediates in the Bacterial Oxidation of Mn(Ⅱ).Proceedings of the National Academy of Sciences, 102(15):5558-5563. https://doi.org/10.1073/pnas.0409119102 Webb, S.M., Tebo, B.M., Bargar, J.R., 2005b.Structural Characterization of Biogenic Mn Oxides Produced in Seawater by the Marine Bacillus sp. Strain SG-1.American Mineralogist, 90(8-9):1342-1357. https://doi.org/10.2138/am.2005.1669 Yang, D.S., Wang, M.K., 2001.Syntheses and Characterization of Well-Crystallized Birnessite.Chemistry of Materials, 13(8):2589-2594. https://doi.org/10.1021/cm010010e Yang, L., Cheng, S., Ji, X., et al., 2015a.Investigations into the Origin of Pseudocapacitive Behavior of Mn3O4 Electrodes Using in Operando Raman Spectroscopy.Journal of Materials Chemistry A, 3(14):7338-7344. https://doi.org/10.1039/C5TA00223K Yang, T.Y., Wen, W., Yin, G.Z., et al., 2015b.Introduction of the X-Ray Diffraction Beamline of SSRF.Nuclear Science and Techniques, 25(2):020101-020105. https://doi.org/10.13538/j.1001-8042/nst.26.020101 冯雄汉, 谭文峰, 刘凡, 等, 2003.碱性介质中水钠锰矿的合成与转化.矿物岩石地球化学通报, 22(2):184-187. http://www.cqvip.com/QK/84215X/200302/7822193.html -









 下载:
下载: