A Nanomineral Material from Thermally Treated Low Grade Natural Rhodochrosite
-
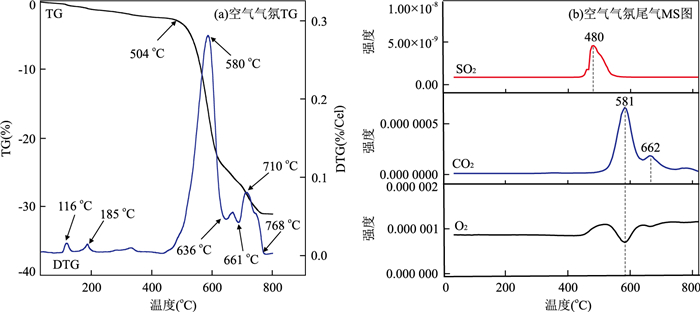
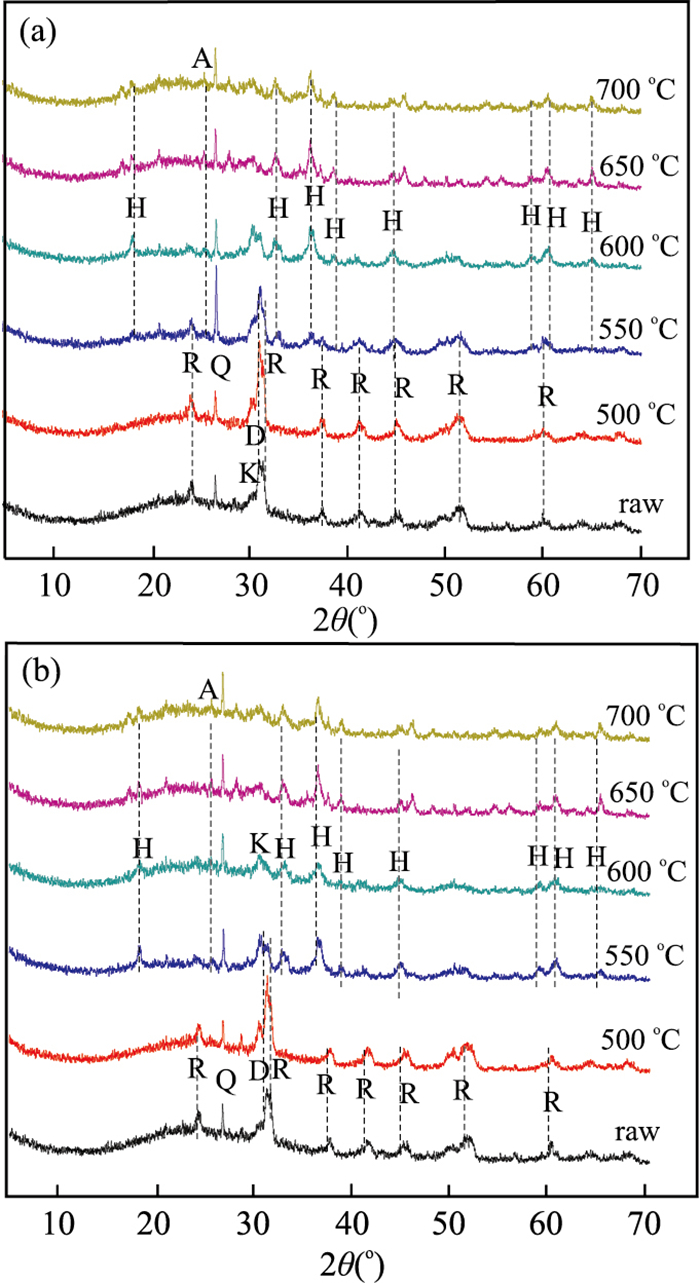
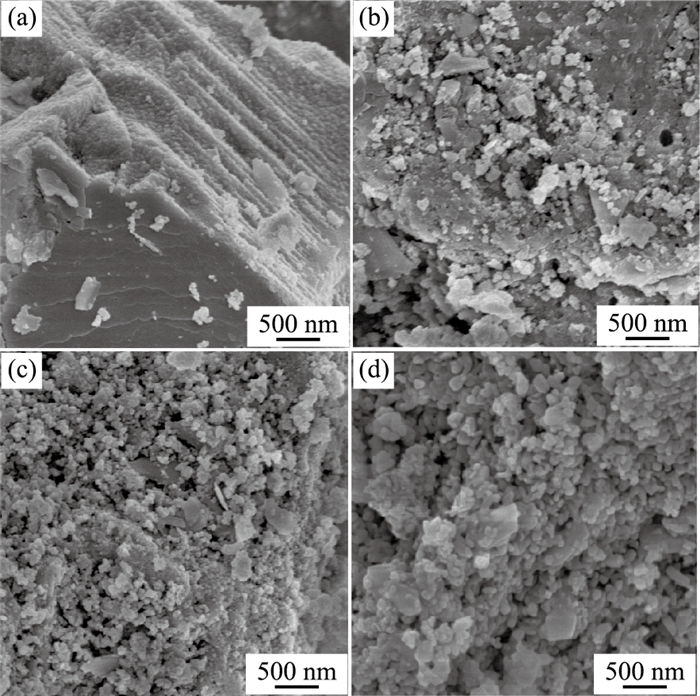
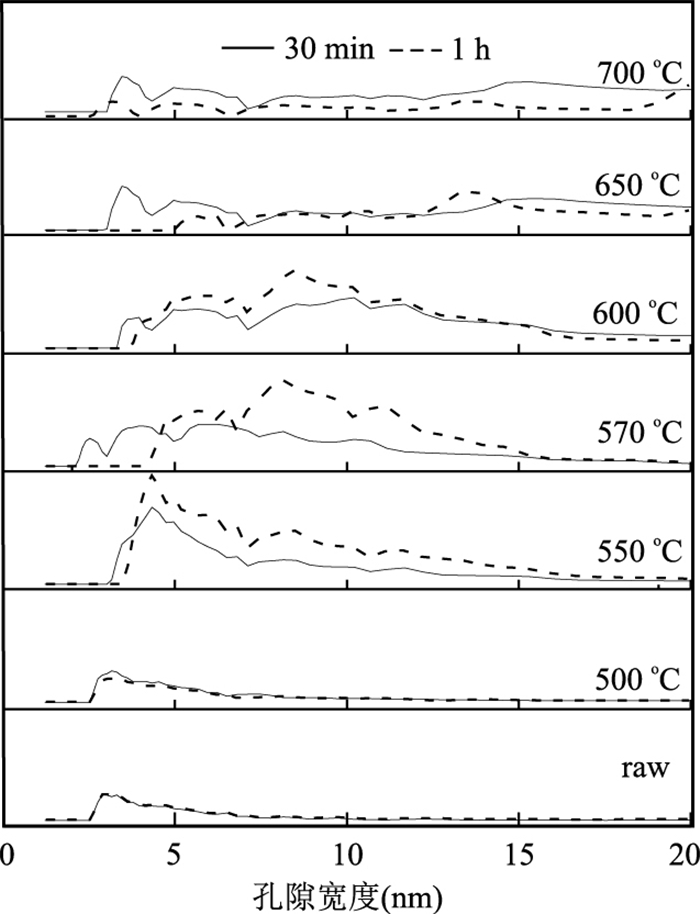
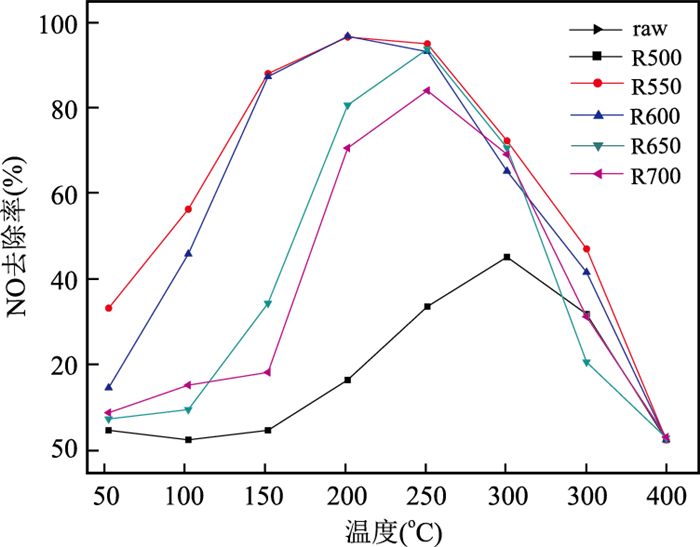
摘要: 通过热处理低品位菱锰矿矿石制备高活性纳米材料,并探究其催化去除NOx、吸附重金属性能.利用X射线荧光光谱仪、透射电子显微镜等研究菱锰矿矿石组成;利用X射线粉末衍射仪、扫描电子显微镜、比表面积分析仪、烟气分析仪、原子吸收分光光度计等研究菱锰矿矿石空气中热处理后结构变化及其NH3-SCR脱硝、重金属吸附效果.菱锰矿矿石主要组分为菱锰矿,含有少量黄铁矿、石英、白云石及硫酸盐.在空气中550℃煅烧1 h后,菱锰矿分解完全,产物以黑锰矿为主晶相,同时含有其他低结晶态锰氧化物;样品表面出现大量3~7 nm气孔,比表面积达到最大(31.5 m2/g).脱硝实验显示R550在170℃时脱硝效率可达到90%;吸附实验表明R550对Cd2+、Pb2+、Cu2+均有较好的吸附作用,表明低品位菱锰矿矿石在空气中550℃煅烧可获得高比表面积、高活性的纳米结构化材料,在环境污染物去除方面有潜在的利用价值.Abstract: A nanomineral material was prepared from thermally treated low grade natural rhodochrosite in this study. The composition of natural rhodochrosite was investigated by combining XRF, TEM and chemical method. The substance and structure after calcination were characterized by TG-MS, XRD, SEM, BET, and XPS. The results show that the mineral constituents of the rhodochrosite ore are rhodochrosite, quartz, pyrite and sulfate oxidized from pyrite. When the temperature increases to 550℃, the rhodochrosite calcines gradually, releasing a great amount of CO2, forming hausmannite and other species of MnOx. As a result, the porous structure (massive accumulation) occurs on the surface of rhodochrosite, which has the pore sizes in 3-7 nanometer, in particular, the maximum specific surface area is 31.5 m2/g. NH3-SCR results show that the sample R550 exhibited a high denitration performance. R550 also has a good removal efficiency of cadmium, lead and copper ions. It is indicated that the nano-porous material with better specific surface area can be prepared through calcining rhodochrosite ore at 550℃.
-
Key words:
- rhodochrosite /
- heat treatment /
- hausmannite /
- nano-mineral /
- specific surface area /
- heavy metal /
- environmental geology
-
表 1 菱锰矿矿石主要成分及含量
Table 1. Mineral and composition of natural rhodochrosite
成分 碳酸盐矿物 黄铁矿 硫酸盐 有机物 黏土 石英 含量(%) 83.7 5.1 1.3 0.4 3.1 6.4 表 2 菱锰矿矿石TEM能谱分析结果
Table 2. TEM-EDS results of natural rhodochrosite
分析点号 Mn Ca Fe Mg 晶体化学式 矿物名称 1 0.55 0.21 0.14 0.09 Mn0.55Ca0.21Mg0.09Fe0.14CO3 含钙菱锰矿 2 0.78 0.13 0.02 0.07 Mn0.78Ca0.13Mg0.07Fe0.02CO3 含钙菱锰矿 3 0.76 0.15 0.02 0.07 Mn0.76Ca0.15Mg0.07Fe0.02CO3 含钙菱锰矿 4 0.67 0.20 0.04 0.09 Mn0.67Ca0.20Mg0.04Fe0.04CO3 含钙菱锰矿 5 0.00 0.50 0.00 0.50 Ca0.5Mg0.5CO3 白云石 表 3 不同价态锰含量
Table 3. Conent of Mnn+
样品 Mn2+(%) Mn3+(%) Mn4+(%) 菱锰矿(℃) 51.3 35.0 13.7 550 39.4 42.4 18.2 700 35.9 48.8 15.2 表 4 菱锰矿不同温度及不同时间煅烧样品的比表面积
Table 4. Specific surface area of natural rhodochrosite and annealed products at different temperatures and time
样品号 表面积(m2/g) 煅烧30 min 煅烧1 h 菱锰矿(℃) 4.6 4.6 400 5.3 5.4 500 7.3 4.7 520 10.1 8.6 550 21.0 31.5 570 25.9 22.8 600 21.1 25.4 650 18.6 14.5 700 16.2 13.9 表 5 去除Pb2+、Cd2+、Cu2+的吸附等温模型参数
Table 5. Sorption isotherm parameters for removal of Pb2+, Cd2+ and Cu2+
Langmuir Qm(mg·g-1) KL(L·mg-1) R2 Pb2+ 343.6 1.658 0.980 Cd2+ 261.1 0.125 0.971 Cu2+ 208.6 0.114 0.993 表 6 不同吸附材料对镉、铅、铜离子吸附能力对比
Table 6. Comparison of adsorption capacities of different adsorption materials for Pb2+, Cd2+ and Cu2+
吸附物 吸附剂 适宜pH T(℃) 吸附容量(mg·g-1) 参考文献 Pd2+ ZnO with montmorillonite 5.0 室温 88.5 Sani et al.(2017) Magnetic chitosan/graphene oxide 5.0 30 79.0 Wang et al.(2016) MnO2/CNTs 7.0 50 78.7 Wang et al.(2007) R550 5.0 20 343.6 本研究 Cd2+ KMnO4-treated biomass 5.0 22 28.1 Wang et al.(2015) Nano-Pumice 6.0 25 200 Khorzughy et al.(2015) 芝麻 6.0 25 84.0 Cheraghi et al.(2015) R550 6.0 20 261.1 本研究 Cu2+ Soybean straw char 5.0 室温 172.0 Tong et al.(2011) Porphyra tenera 5.5 20 75.1 Park et al.(2016) 活性炭纤维 4.0 室温 177.1 Huang and Su(2010) R550 5.0 20 208.6 本研究 -
Bai, Y., Jefferson, W.A., Liang, J., et al., 2017.Antimony Oxidation and Adsorption by In-Situ Formed Biogenic Mn Oxide and Fe-Mn Oxides.Journal of Environmental Sciences, 54:126-134. doi: 10.1016/j.jes.2016.05.026 Chen, H.F., Xia, Y., Huang, H., et al., 2017.Highly Dispersed Surface Active Species of Mn/Ce/Tiw Catalysts for High Performance at Low Temperature NH3-SCR.Chemical Engineering Journal, 330:1195-1202. doi: 10.1016/j.cej.2017.08.069 Cheraghi, E., Ameri, E., Moheb, A., 2015.Adsorption of Cadmium Ions from Aqueous Solutions Using Sesame as a Low-Cost Biosorbent:Kinetics and Equilibrium Studies.International Journal of Environmental Science & Technology, 12(8):1-14. http://en.journals.sid.ir/ViewPaper.aspx?ID=482558 Delimaris, D., Ioannides, T., 2008.VOC Oxidation over MnOx-CeO2 Catalysts Prepared by a Combustion Method.Applied Catalysis B:Environmental, 84(1-2):303-312. doi: 10.1016/j.apcatb.2008.04.006 Du, X.L., Han, Q., Li, J.Q., et al., 2017.The Behavior of Phosphate Adsorption and Its Reactions on the Surfaces of Fe-Mn Oxide Adsorbent.Journal of the Taiwan Institute of Chemical Engineers, 76:167-175. doi: 10.1016/j.jtice.2017.04.023 Ettireddy, P.R., Ettireddy, N., Mamedov, S., et al., 2007.Surface Characterization Studies of TiO2 Supported Manganese Oxide Catalysts for Low Temperature SCR of NO with NH3.Applied Catalysis B:Environmental, 76(1-2):123-134. doi: 10.1016/j.apcatb.2007.05.010 Fang, D., Xie, J.L., Hu, H., et al., 2015.Identification of MnOx Species and Mn Valence States in MnOx/TiO2 Catalysts for Low Temperature SCR.Chemical Engineering Journal, 271:23-30. doi: 10.1016/j.cej.2015.02.072 Hagelstein, K., 2009.Globally Sustainable Manganese Metal Production and Use.Journal of Environmental Management, 90(12):3736-3740. doi: 10.1016/j.jenvman.2008.05.025 Huang, C.C., Su, Y.J., 2010.Removal of Copper Ions from Wastewater by Adsorption/Electrosorption on Modified Activated Carbon Cloths.Journal of Hazardous Materials, 175(1-3):477-483. doi: 10.1016/j.jhazmat.2009.10.030 Johnson, J.E., Webb, S.M., Ma, C., et al., 2016.Manganese Mineralogy and Diagenesis in the Sedimentary Rock Record.Geochimica et Cosmochimica Acta, 173:210-231. doi: 10.1016/j.gca.2015.10.027 Kashiwabara, T., Kubo, S., Tanaka, M., et al., 2017.Stable Isotope Fractionation of Tungsten during Adsorption on Fe and Mn (Oxyhydr) Oxides.Geochimica et Cosmochimica Acta, 204:52-67. doi: 10.1016/j.gca.2017.01.031 Khorzughy, S.H., Eslamkish, T., Ardejani, F, D., et al., 2015.Cadmium Removal from Aqueous Solutions by Pumice and Nano-Pumice.Korean J.Chem.Eng., 32(1):88-96. doi: 10.1007/s11814-014-0168-2 Lee, J.H., Kennedy, D.W., Dohnalkova, A., et al., 2011.Manganese Sulfide Formation via Concomitant Microbial Manganese Oxide and Thiosulfate Reduction.Environmental Microbiology, 13(12):3275-3288. doi: 10.1111/j.1462-2920.2011.02587.x Li, Q., Liu, H.B., Chen, T.H., et al., 2017.NH3-SCR Performance of Nano-α-Fe2O3 Derived from Thermally Treated Siderite.Acta Scientiae Circumstantiae, 37(7):2482-2489 (in Chinese with English abstract). Li, Q.L., Yi, H.S., Xia, G.Q., et al., 2017.Characteristics and Implication of Carbon and Oxygen Isotopes in Ga-Rich Manganese-Bearing Rock Series in Dongping, Guangxi.Earth Science, 42(9):1508-1518 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-DQKX801.021.htm Li, X.Q., Zhou, L.P., Gao, J., et al., 2009.Synthesis of Mn3O4 Nanoparticles and Their Catalytic Applications in Hydrocarbon Oxidation.Powder Technology, 190(3):324-326. doi: 10.1016/j.powtec.2008.08.010 Ministry of Land and Resources of the People's Republic of China, 2016.China Mineral Resources.Geological Publishing House, Beijing, 4-6 (in Chinese). Park, S.H., Cho, H.J., Ryu, C., et al., 2016.Removal of Copper(Ⅱ) in Aqueous Solution Using Pyrolytic Biochars Derived from Red Macroalga Porphyra Tenera.Journal of Industrial and Engineering Chemistry, 36:314-319. doi: 10.1016/j.jiec.2016.02.021 Pereira, M.J., Lima, M.M.F., Lima, R.M.F., 2014.Calcination and Characterisation Studies of a Brazilian Manganese Ore Tailing.International Journal of Mineral Processing, 131:26-30. doi: 10.1016/j.minpro.2014.08.003 Ptáek, P., Bartoníková, E., Švec, J., et al., 2015.The Kinetics and Mechanism of Thermal Decomposition of SrCO3 Polymorphs.Ceramics International, 41(1):115-126. doi: 10.1016/j.ceramint.2014.08.043 Sani, H.A., Ahmad, M.B., Hussein, M.Z., et al., 2017.Nanocomposite of ZnO with Montmorillonite for Removal of Lead and Copper Ions from Aqueous Solutions.Process Safety and Environmental Protection, 109:97-105. doi: 10.1016/j.psep.2017.03.024 Tong, X.J., Li, J.Y., Yuan, J.H., et al., 2011.Adsorption of Cu(Ⅱ) by Biochars Generated from Three Crop Straws.Chemical Engineering Journal, 172(2-3):828-834. doi: 10.1016/j.cej.2011.06.069 Wang, H., Gao, B., Wang, S., et al., 2015.Removal of Pb(Ⅱ), Cu(Ⅱ), and Cd(Ⅱ) from Aqueous Solutions by Biochar Derived from KMnO4 Treated Hickory Wood.Bioresource Technology, 197:356-362. doi: 10.1016/j.biortech.2015.08.132 Wang, L.B., Jiu, K., Zeng, W.T., et al., 2013.Characteristics of Lower Cambrian Marine Black Shales and Evaluation of Shale Gas Prospective Area in Qianbei Area, Upper Yangtze Region.Acta Petrologica Sinica, 29(9):3263-3278 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-YSXB201309024.htm Wang, S., Gong, W., Liu, X., et al., 2007.Removal of Lead(Ⅱ) from Aqueous Solution by Adsorption onto Manganese Oxide-Coated Carbon Nanotubes.Separation and Purification Technology, 58(1):17-23. doi: 10.1016/j.seppur.2007.07.006 Wang, Y., Li, L., Luo, C., et al., 2016.Removal of Pb2+ from Water Environment Using a Novel Magnetic Chitosan/Graphene Oxide Imprinted Pb2+.International Journal of Biological Macromolecules, 86:505-511. doi: 10.1016/j.ijbiomac.2016.01.035 Xing, B.B., Chen, T.H., Liu, H.B., et al., 2016a.Removal of Low Concentration Phosphate Using Low Grade Siderite.Journal of the Chinese Ceramic Society, 44(2):299-307 (in Chinese with English abstract). Xing, B.B., Chen, T.H., Qing, C.S., et al., 2016b.Structural Characteristic of Natural Siderite during Thermal Treatment.Journal of the Chinese Ceramic Society, 44(8):1207-1212 (in Chinese with English abstract). http://www.en.cnki.com.cn/Article_en/CJFDTotal-GXYB201608019.htm Xie, J.J., Chen, T.H., Xing, B.B., et al., 2016.The Thermochemical Activity of Dolomite Occurred in Dolomite-Palygorskite.Applied Clay Science, 119:42-48. doi: 10.1016/j.clay.2015.07.014 Yang, S.J., Wang, C.Z., Li, J.H., et al., 2011.Low Temperature Selective Catalytic Reduction of No with NH3 over Mn-Fe Spinel:Performance, Mechanism and Kinetic Study.Applied Catalysis B:Environmental, 110:71-80. doi: 10.1016/j.apcatb.2011.08.027 Yang, Y., Chen, T.H., Sumona, M., et al., 2017.Utilization of Iron Sulfides for Wastewater Treatment:A Critical Review.Reviews in Environmental Science and Bio/Technology, 16(2):289-308. doi: 10.1007/s11157-017-9432-3 Yao, X.J., Kong, T.T., Yu, S.H., et al., 2017.Influence of Different Supports on the Physicochemical Properties and Denitration Performance of the Supported Mn-Based Catalysts for NH3-SCR at Low Temperature.Applied Surface Science, 402:208-217. doi: 10.1016/j.apsusc.2017.01.081 Zhang, F.F., Zhu, X.K., Gao, Z.F., et al., 2013.Implication of the Precipitation Mode of Manganese and Ultra-High δ34S Values of Pyrite in Mn-Carbonate of Xixibao Mn Ore Deposit in Northeastern Guizhou Province.Geological Review, 59(2):274-286 (in Chinese with English abstract). Zhang, J., Li, R.H., Li, J., et al., 2013.Removal Efficiency of Phosphorus by Pyrite.Chinese Journal of Environmental Engineering, 7(10):3856-3860 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-HJJZ201310028.htm Zhang, Z.J., Zhou, D.B., Zhang, Q., et al., 2010.Preparation and Characterization of High Purity Chemical Manganese Dioxille.Applied Chemical Industry, 39(9):1359-1363 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-SXHG201009025.htm Zheng, Y.J., Liu, Z.C., 2011.Preparation of Monodispersed Micaceous Iron Oxide Pigment from Pyrite Cinders.Powder Technology, 207(1-3):335-342. doi: 10.1016/j.powtec.2010.11.015 Zou, X.H., Chen, T.H., Liu, H.B., et al., 2016.Catalytic Cracking of Toluene over Hematite Derived from Thermally Treated Natural Limonite.Fuel, 177:180-189. doi: 10.1016/j.fuel.2016.02.094 Zou, X.H., Chen, T.H., Zhang, P., et al., 2013.Structural Characteristic of Natural Goethite by Thermal Treatment.Journal of the Chinese Ceramic Society, 41(10):1440-1446 (in Chinese with English abstract). http://www.en.cnki.com.cn/Article_en/CJFDTOTAL-GXYB201310021.htm 李骞, 刘海波, 陈天虎, 等, 2017.煅烧菱铁矿制备纳米结构化Fe2O3及其NH3-SCR脱硝活性研究.环境科学学报, 37(7):2482-2489. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkxxb201707009 李启来, 伊海生, 夏国清, 等, 2017.广西东平富Ga含锰岩系碳、氧同位素特征及意义.地球科学, 42(9):1508-1518. http://earth-science.net/WebPage/Article.aspx?id=3648 中华人民共和国国土资源部, 2016.中国矿产资源报告.北京:地质出版社, 4-6. 王丽波, 久凯, 曾维特, 等, 2013.上扬子黔北地区下寒武统海相黑色泥页岩特征及页岩气远景区评价.岩石学报, 29(9):3263-3278. http://www.ysxb.ac.cn/ysxb/ch/reader/download_pdf.aspx?file_no=20130924&year_id=2013&quarter_id=9&falg=1 邢波波, 陈天虎, 刘海波, 等, 2016a.用低品位菱铁矿石去除水中低浓度磷酸盐.硅酸盐学报, 44(2):299-307. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gsyxb201602017 邢波波, 陈天虎, 庆承松, 等, 2016b.天然菱铁矿在热处理过程中的结构变化.硅酸盐学报, 44(8):1207-1212. http://www.cnki.com.cn/Article/CJFDTOTAL-XTKY200201014.htm 张飞飞, 朱祥坤, 高兆富, 等, 2013.黔东北西溪堡锰矿的沉淀形式与含锰层位中黄铁矿异常高Δ34S值的成因.地质论评, 59(2):274-286. http://www.cqvip.com/QK/91067X/201302/45527793.html 张菁, 李睿华, 李杰, 等, 2013.天然黄铁矿的除磷性能.环境工程学报, 7(10):3856-3860. http://www.cnki.com.cn/Article/CJFDTotal-HJJZ201310028.htm 章泽杰, 周德璧, 张清, 等, 2010.二氧化锰的碳酸锰热解制备及其对氧还原催化性能研究.应用化工, 39(9):1360-1363. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sxhg201009026 邹雪华, 陈天虎, 张萍, 等, 2013.天然针铁矿热处理产物的结构特征.硅酸盐学报, 41(10):1442-1446. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gsyxb201310021 -










 下载:
下载: