Study on Adsorption Behavior and Mechanism of Shale Gas by Using GCMC Molecular Simulation
-
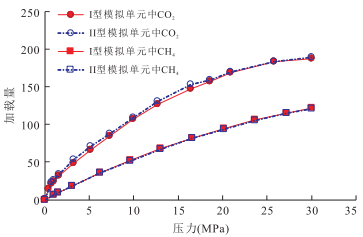
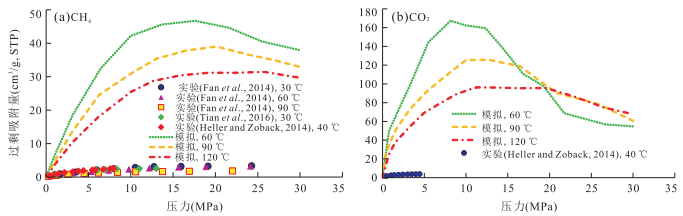
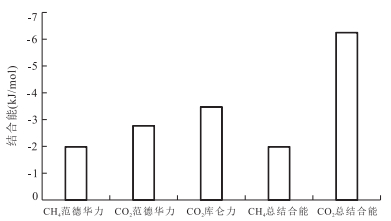
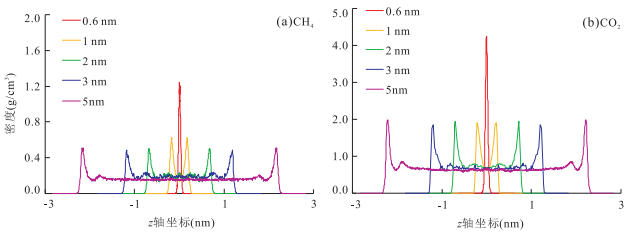
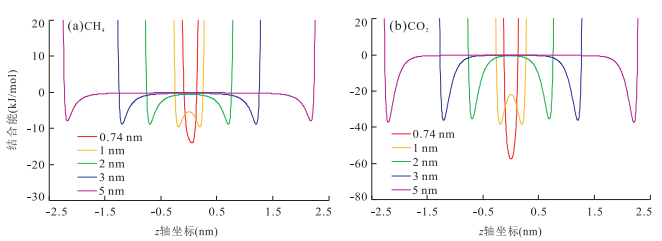
摘要: 揭示页岩气的吸附机理是阐明页岩气的吸附规律及转化条件、建立具有普适意义的定量评价模型的基础.采用GCMC(Grand Canonical Monte Carlo)分子模拟方法,对不同温压条件下CH4和CO2在不同孔径的伊利石狭缝形孔隙中的吸附行为进行模拟,结果表明,分子模拟与实验所得的吸附量归一化到单位表面积才具有相同的内涵和比较的意义.在此基础上进行的对比表明,分子模拟与实验结果相近,奠定了由分子模拟考察页岩气吸附行为和机理的基础:气体吸附于矿物表面的内因(机理)是气-固分子之间的范德华力和库仑力,伊利石表面对CO2的吸附能力比其对CH4的吸附能力强是其结合能更高的反映;CH4和CO2在伊利石表面的吸附虽然并非严格的单分子层吸附,但以一个强吸附层为主;孔径减小到微孔后吸附相密度将发生叠加,形成微孔填充,也是其结合能叠加的结果.Abstract: The mechanism of shale gas adsorption is the theoretical foundation for elucidating the adsorption and transformation conditions, and establishing a universal quantitative evaluation model. The adsorption behavior of CH4 and CO2 in illite slit pores with different sizes under various temperature and pressure conditions was simulated by GCMC (Grand Canonical Monte Carlo) method. It is found that adsorption capacities obtained from both molecular simulation and experiments have the same connotations and are comparable when being normalized to the surface area, under which conditions the molecular simulation results are consistent with the experimental measurements. In this way, the basis for the study of adsorption behavior and mechanism of shale gas is established by molecular simulation:the internal cause (mechanism) of gas adsorption on the mineral surface is van der Waals force and Coulomb force in gas-solid molecules, the larger adsorption capacity of CO2 than CH4 on the surface of the illite is the reflection of a higher binding energy; the adsorption of CH4 and CO2 on the illite is not rigorous monolayer-adsorption, but a strong adsorption layer mainly; the adsorption phase density will overlap when pore size reduced to micropore, and microporous filling is thus formed, which is also a result from the superposition of their binding energy.
-
表 1 Ⅰ型、Ⅱ型钾伊利石模拟单元比表面积以及CH4和CO2气体分子动力学直径统计
Table 1. Kinetic diameters of both CH4 and CO2 molecules and specific surface areas of types Ⅰ and Ⅱ K-illite simulation cells
吸附质 动力学直径(nm) 模拟单元类型 比表面积(m2/g) CH4 0.38 Ⅰ 997.73 Ⅱ 529.60 CO2 0.33 Ⅰ 989.79 Ⅱ 536.22 -
Badics, B., Vetö, I., 2012.Source Rocks and Petroleum Systems in the Hungarian Part of the Pannonian Basin:The Potential for Shale Gas and Shale Oil Plays.Marine & Petroleum Geology, 31(1):53-69. https://doi.org/10.1016/j.marpetgeo.2011.08.015 Brunauer, S., Emmett, P.H., Teller, E., 1938.Adsorption of Gases in Multimolecular Layers.Journal of American Chemical Society, 60(2):309-319. https://doi.org/10.1021/ja01269a023 Chen, G., Lu, S., Zhang, J., et al., 2016a.Research of CO2 and N2 Adsorption Behavior in K-Illite Slit Pores by GCMC Method.Scientific Reports, 6:37579. https://doi.org/10.1038/srep37579 Chen, G., Zhang, J., Lu, S., et al., 2016b.Adsorption Behavior of Hydrocarbon on Illite.Energy & Fuels, 30(11):9114-9121. https://doi.org/10.1021/acs.energyfuels.6b01777 Connolly, M.L., 1983a.Analytical Molecular Surface Calculation.Journal of Applied Crystallography, 16(5):548-558. https://doi.org/10.1107/s0021889883010985 Connolly, M.L., 1983b.Solvent-Accessible Surfaces of Proteins and Nucleic Acids.Science, 221(4621):709-713. https://doi.org/10.1126/science.6879170 Curtis, J.B., 2002.Fractured Shale-Gas Systems.AAPG Bulletin, 86(11):1921-1938. https://doi.org/10.1306/61EEDDBE-173E-11D7-8645000102C1865D Cygan, R.T., Liang, J.J., Kalinichev, A.G., 2004.Molecular Models of Hydroxide, Oxyhydroxide, and Clay Phases and the Development of a General Force Field.Journal of Physical Chemistry B, 108(4):1255-1266. https://doi.org/10.1021/jp0363287 Dubinin, M.M., 1960.The Potential Theory of Adsorption of Gases and Vapors for Adsorbents with Energetically Nonuniform Surfaces.Chemical Reviews, 60(2):235-241. https://doi.org/10.1021/cr60204a006 Dubinin, M.M., 1975.Physical Adsorption of Gases and Vapors in Micropores.Progress in Surface and Membrane Science, 9:1-70. https://doi.org/10.1016/B978-0-12-571809-7.50006-1 Fan, E., Tang, S.H., Zhang, C.L., et al., 2014.Methane Sorption Capacity of Organics and Clays in High-over Matured Shale-Gas Systems.Energy Exploration & Exploitation, 32(6):927-942. https://doi.org/10.1260/0144-5987.32.6.927 Gasparik, M., Rexer, T.F.T., Aplin, A.C., et al., 2014.First International Inter-Laboratory Comparison of High-Pressure CH4, CO2, and C2H6, Sorption Isotherms on Carbonaceous Shales.International Journal of Coal Geology, 132:131-146. https://doi.org/10.1016/j.coal.2014.07.010 Harries, J.E., 1970.The Quadrupole Moment of CO2, Measured from the Far Infrared Spectrum.Journal of Physics B:Atomic and Molecular Physics, 3(12):L150-L152. https://doi.org/10.1088/0022-3700/3/12/021 Heller, R., Zoback, M., 2014.Adsorption of Methane and Carbon Dioxide on Gas Shale and Pure Mineral Samples.Journal of Unconventional Oil & Gas Resources, 8:14-24. https://doi.org/10.1016/j.juogr.2014.06.001 Ji, L., Zhang, T., Milliken, K.L., et al., 2012.Experimental Investigation of Main Controls to Methane Adsorption in Clay-Rich Rocks.Applied Geochemistry, 27(12):2533-2545. https://doi.org/10.1016/j.apgeochem.2012.08.027 Jiang, S., Tang, X.L., Steve, O., et al., 2017.Enrichment Factors and Current Misunderstanding of Shale Oil and Gas:Case Study of Shales in U.S., Argentina and China.Earth Science, 42(7):1083-1091 (in Chinese with English abstract). https://doi.org/10.3799/dqkx.2017.087 Jin, Z.H., Firoozabadi, A., 2013.Methane and Carbon Dioxide Adsorption in Clay-Like Slit Pores by Monte Carlo Simulations.Fluid Phase Equilibria, 360(1):456-465. https://doi.org/10.1016/j.fluid.2013.09.047 Jin, Z.H., Firoozabadi, A., 2014.Effect of Water on Methane and Carbon Dioxide Sorption in Clay Minerals by Monte Carlo Simulations.Fluid Phase Equilibria, 382:10-20. https://doi.org/10.1016/j.fluid.2014.07.035 Jorgensen, W.L., Madura, J.D., Swenson, C.J., 1984.Optimized Intermolecular Potential Functions for Liquid Hydrocarbons.J.Am.Chem.Soc.(United States), 106(22):6638-6646. https://doi.org/10.1021/ja00334a030 Krooss, B.M., Bergen, F.V., Gensterblum, Y., et al., 2002.High-Pressure Methane and Carbon Dioxide Adsorption on Dry and Moisture-Equilibrated Pennsylvanian Coals.International Journal of Coal Geology, 51(2):69-92. https://doi.org/10.1016/S0166-5162(02)00078-2 Lee, J.H., Guggenheim, S., 1981.Single-Crystal X-Ray Refinement of Pyrophyllite-1Tc.American Mineralogist, 66(3-4):350-357. https://pubs.geoscienceworld.org/msa/ammin/article/66/3-4/350/41261/single-crystal-x-ray-refinement-of-pyrophyllite Liu, Y., Wilcox, J., 2012.Molecular Simulation of CO2 Adsorption in Micro-and Mesoporous Carbons with Surface Heterogeneity.International Journal of Coal Geology, 104(1):83-95. https://doi.org/10.1016/j.coal.2012.04.007 Liu, Y., Zhu, Y.M., Li, W., et al., 2016.Molecular Simulation of Methane Adsorption in Shale Based on Grand Canonical Monte Carlo Method and Pore Size Distribution.Journal of Natural Gas Science & Engineering, 30:119-126. https://doi.org/10.1016/j.jngse.2016.01.046 Macht, F., Eusterhues, K., Pronk, G.J., et al., 2011.Specific Surface Area of Clay Minerals:Comparison between Atomic Force Microscopy Measurements and Bulk-Gas (N2) and -Liquid (EGME) Adsorption Methods.Applied Clay Science, 53(1):20-26. https://doi.org/10.1016/j.clay.2011.04.006 Macht, F., Totsche, K.U., Eusterhues, K., et al., 2010.Topography and Surface Properties of Clay Minerals Analyzed by Atomic Force Microscopy.Proceedings of the 19th World Congress of Soil Science:Soil Solutions for a Changing World, Brisbane, 206-209. https://iuss.org/19th%20WCSS/Symposium/pdf/2384.pdf Mosher, K., He, J., Liu, Y., et al., 2013.Molecular Simulation of Methane Adsorption in Micro-and Mesoporous Carbons with Applications to Coal and Gas Shale Systems.International Journal of Coal Geology, 109-110(2):36-44. https://doi.org/10.1016/j.coal.2013.01.001 Potoff, J.J., Siepmann, J.I., 2001.Vapor-Liquid Equilibria of Mixtures Containing Alkanes, Carbon Dioxide, and Nitrogen.Aiche Journal, 47(7):1676-1682. https://doi.org/10.1002/aic.690470719 Refson, K., Park, S.H., Sposito, G., 2003.Ab Initio Computational Crystallography of 2:1 Clay Minerals:1.Pyrophyllite-1Tc.The Journal of Physical Chemistry B, 107(48):13376-13383. https://doi.org/10.1021/jp0347670 Ross, D.J.K., Bustin, R.M., 2009.The Importance of Shale Composition and Pore Structure upon Gas Storage Potential of Shale Gas Reservoirs.Marine & Petroleum Geology, 26(6):916-927. https://doi.org/10.1016/j.marpetgeo.2008.06.004 Sun, R.Y., Zhang, Y.F., Fan, K.K., et al., 2015.Molecular Simulations of Adsorption Characteristics of Clay Minerals in Shale.CIESC Journal, 66(6):2118-2122 (in Chinese with English abstract). https://doi.org/10.11949/j.issn.0438-1157.20141766 Tan, J., Weniger, P., Krooss, B., et al., 2014.Shale Gas Potential of the Major Marine Shale Formations in the Upper Yangtze Platform, South China, Part Ⅱ:Methane Sorption Capacity.Fuel, 129(4):204-218. https://doi.org/10.1016/j.fuel.2014.03.064 Tian, H., Zhang, S.C., Liu, S.B., et al., 2016.The Dual Influence of Shale Composition and Pore Size on Adsorption Gas Storage Mechanism of Organic-Rich Shale.Natural Gas Geoscience, 27(3):494-502. https://doi.org/10.11764/j.issn.1672-1926.2016.03.0494 Wang, S., Javadpour, F., Feng, Q., 2016.Molecular Dynamics Simulations of Oil Transport through Inorganic Nanopores in Shale.Fuel, 171:74-86. https://doi.org/10.1016/j.fuel.2015.12.071 Xiang, J.H., Zeng, F.G., Liang, H.Z., et al., 2014.Molecular Simulation of the CH4/CO2/H2O Adsorption onto the Molecular Structure of Coal.Science China Earth Sciences, 44(7):1418-1428 (in Chinese). https://doi.org/10.1007/s11430-014-4849-9 Yang, N.N., Liu, S.Y., Yang, X.N., 2015.Molecular Simulation of Preferential Adsorption of CO2 over CH4 in Na-Montmorillonite Clay Material.Applied Surface Science, 356:1262-1271. https://doi.org/10.1016/j.apsusc.2015.08.101 Zhang, J.F., Clennell, M.B., Dewhurst, D.N., et al., 2014.Combined Monte Carlo and Molecular Dynamics Simulation of Methane Adsorption on Dry and Moist Coal.Fuel, 122(15):186-197. https://doi.org/10.1016/j.fuel.2014.01.006 Zhang, T.W., Ellis, G.S., Ruppel, S.C., et al., 2012.Effect of Organic-Matter Type and Thermal Maturity on Methane Adsorption in Shale-Gas Systems.Organic Geochemistry, 47(6):120-131. https://doi.org/10.1016/j.orggeochem.2012.03.012 Zhang, X.M., Shi, W.Z., Shu, Z.G., et al., 2017.Calculation Model of Shale Gas Content and Its Application in Fuling Area.Earth Science, 42(7):1157-1168 (in Chinese with English abstract). https://doi.org/10.3799/dqkx.2017.094 Zhang, X.M., Shi, W.Z., Xu, Q.H., et al., 2015.Reservoir Characteristics and Controlling Factors of Shale Gas in Jiaoshiba Area, Sichuan Basin.Acta Petrolei Sinica, 36(8):926-939, 953 (in Chinese with English abstract). https://doi.org/10.7623/syxb201508004 蒋恕, 唐相路, Steve, O., 等, 2017.页岩油气富集的主控因素及误辩:以美国、阿根廷和中国典型页岩为例.地球科学, 42(7):1083-1091. http://www.earth-science.net/WebPage/Article.aspx?id=3609 孙仁远, 张云飞, 范坤坤, 等, 2015.页岩中黏土矿物吸附特性分子模拟.化工学报, 66(6):2118-2122. http://www.cnki.com.cn/Article/CJFDTotal-HGSZ201506018.htm 相建华, 曾凡桂, 梁虎珍, 等, 2014.CH4/CO2/H2O在煤分子结构中吸附的分子模拟.中国科学:地球科学, 44(7):1418-1428. http://xueshu.baidu.com/s?wd=paperuri%3A%2856e1bfe054180687e7094d8d527f71e6%29&filter=sc_long_sign&tn=SE_xueshusource_2kduw22v&sc_vurl=http%3A%2F%2Fkns.cnki.net%2FKCMS%2Fdetail%2Fdetail.aspx%3Ffilename%3Djdxk201407006%26dbname%3DCJFD%26dbcode%3DCJFQ&ie=utf-8&sc_us=10315646226932109291 张晓明, 石万忠, 舒志国, 等, 2017.涪陵地区页岩含气量计算模型及应用.地球科学, 42(7):1157-1168. http://www.earth-science.net/WebPage/Article.aspx?id=3602 张晓明, 石万忠, 徐清海, 等, 2015.四川盆地焦石坝地区页岩气储层特征及控制因素.石油学报, 36(8):926-939, 953. doi: 10.7623/syxb201508004 -










 下载:
下载: