Analytical Method for δ18O of Phosphate in Trace Apatite
-
摘要: 生物磷灰石壳体的磷酸根氧同位素组成是重建古温度理想指标之一,在古环境研究中具有重要意义.针对牙形石等磷灰石量极少的情况,稳定可靠的前处理方法是分析其δ18OPO4的重要保障,目前仅有少数国外实验室已建立了相关提取分析方法.结合这些方法的优缺点对分析步骤进行改进优化,建立了微量磷灰石的磷酸根氧同位素分析方法,通过硝酸消解磷灰石并除去非磷酸根氧,利用KF溶液沉淀法分离Ca2+,采用氨缓冲溶液形式调节pH,并加入AgNO3溶液以氨挥发法将PO43-转化成Ag3PO4结晶分离,气体稳定同位素质谱仪在线测定Ag3PO4氧同位素组成.结果表明,方法全流程未产生明显的氧同位素分馏,样品最低仅需0.2 mg,标准偏差小于0.2‰(1σ),与目前国际报道的分析精度一致.Abstract: The oxygen isotope composition of phosphate (δ18OPO4) in bioapatite plays a significant role in paleo-environmental research as one of the ideal proxies for paleo-temperature reconstruction. However, for trace bioapatite(e.g., conodonts), a reliable pre-treatment technology is quite important and difficult for its δ18OPO4 analysis, resulting in analytical technique established only in several overseas laboratories. Here we combine the advantages of those methods and present a protocol on the analysis of δ18OPO4 as Ag3PO4 for bioapatite of total sample size as small as 0.2 mg using a thermal conversion elemental analyzer (TC/EA) coupled to a continuous flow isotope ratio mass spectrometer (CF-IRMS) via a helium stream. Ag3PO4 is precipitated by NH3 buffer method after apatite being dissolved with nitric acid, Ca2+ being removed with KF solution and the solution being neutralized with ammonia buffer solution. The results indicate that analysis of δ18OPO4 maintains an external precision of ±0.2‰(1σ), meeting the international analytical standards. This method for analyzing δ18OPO4 of phosphate extracted from bioapatite is robust to be used to reconstruct paleo-temperature.
-
Key words:
- bioapatite /
- oxygen isotope of phosphate /
- analytical method /
- biogeochemistry
-
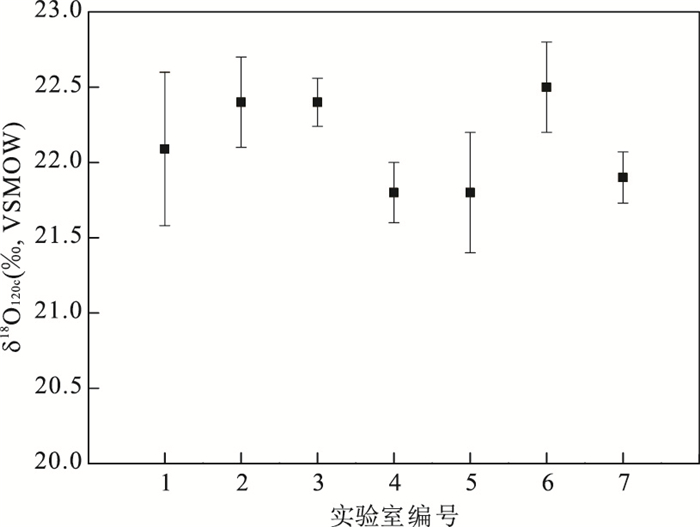
图 6 不同实验室对NBS 120c的δ18OPO4提取测试结果对比
1.Vennemann et al.(2002): 22.09‰±0.51‰; 2.LaPorte et al.(2009): 22.4‰±0.3‰; 3.Joachimski et al.(2009): 22.4‰±0.16‰; 4.Halas et al.(2011): 21.8‰±0.2‰; 5.Rosenau et al.(2014): 21.8‰±0.4‰; 6.Griffin et al.(2015): 22.5‰±0.3‰; 7.本文: 21.9‰±0.17‰(精度1σ)
Fig. 6. δ18OPO4values of NBS 120c from different laboratories and different methodsδ18OPO4values of NBS 120c from different laboratories and different methods
表 1 微量磷灰石中δ18OPO4分析的TC/EA法比较
Table 1. Comparison of TC/EA method for δ18OPO4 analysis of trace apatite
样品量(mg) 1σ(‰) 回收率(%) 流程简介 主要优缺点 参考文献 20
0.51
(氟化法0.09)- NaOCl除有机物,NaOH除腐殖酸,HF溶液溶解磷灰石并沉淀Ca2+,KOH中和溶液,银氨溶液回收PO43-. 流程简单;要求样品量大,精度低,HF对仪器有损害 Vennemann et al., 2002 0.10~0.45 0.15 85~97 0.5 mol/L HNO3溶解磷灰石,阳离子交换树脂除Ca2+,浓氨水中和,AgNO3回收PO43-. 精度高,可分析44Ca、稀土元素等;使用阳离子交换树脂费时费力 LaPorte et al., 2009 0.5~1.0 0.15 92~99 2.0 mol/L HNO3溶解磷灰石,KOH中和,HF沉淀Ca2+,银氨溶液回收PO43-. 精度高;中和工作量大 Joachimski et al., 2009 0.3~0.8 0.10~0.40 20~85 0.5 mol/L HNO3溶解磷灰石,KOH中和,KF沉淀Ca2+,银氨溶液回收PO43-. 方法完整系统;溶液量低难操作,回收率不稳定 Griffin et al., 2015 表 2 相关的仪器、材料和试剂
Table 2. Relative instrument, materials and reagents
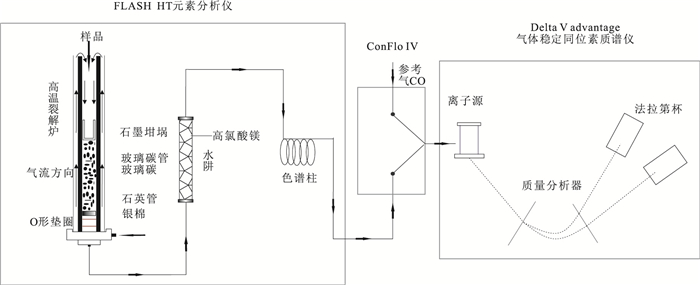
类别 仪器、材料与试剂 测试仪器 Flash HT元素分析仪+Delta V气体稳定同位素比值质谱仪(Thermo Fisher公司) 前处理设备材料 高速离心机;隔膜真空泵抽滤设备一套;移液枪(1 mL);PFA烧杯(5 mL);离心管(2 mL);玻璃纤维滤膜(0.45 μm);阳离子交换树脂(AG50W-X12) 试剂 硝酸、硝酸银、氨水、氢氧化钾和氟化钾(分析纯) 标准样品 磷灰石标样NBS 120c(NIST);磷灰石标样NBS 694(NIST);磷酸银氧同位素标样(Elemental Microanalysis公司) 实际样品 大唇犀、三趾马和羚羊牙齿磷灰石(200目) 表 3 不同消解温度对PO43-回收率的影响
Table 3. PO43- yields in different digestion temperature
样品名称 PO43-回收率(±1σ,n=3,%) 20 ℃ 70 ℃ 150 ℃ NBS 120c 93.9±2.1 94.0±4.1 93.3±2.2 NBS 694 92.8±0.8 93.2±1.1 92.9±1.6 表 4 不同除Ca2+方法对NBS 120c PO43-回收率和δ18OPO4的影响
Table 4. Average PO43- yields and δ18OPO4 values of NBS 120c under different Ca2+ removing options
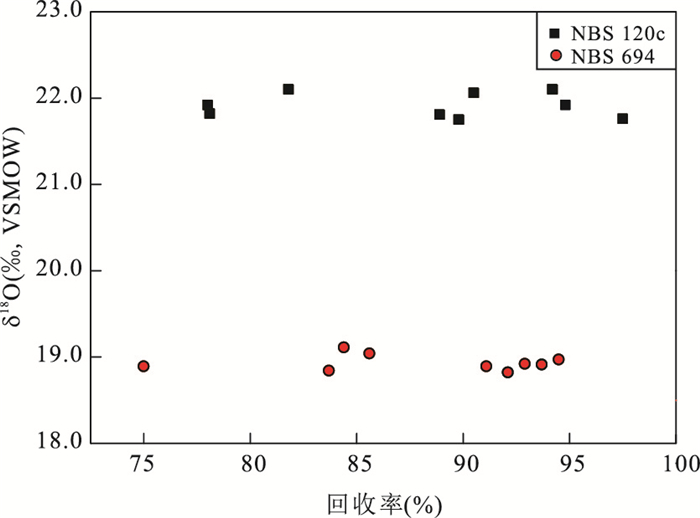
不除Ca2+(n=3) 使用树脂(n=6) 硝酸+KF(n=6) 后加KF(n=6) PO43-回收率(±1σ, %) 45.9±14.3 89.1±1.5 93.4±2.2 91.4±1.9 δ18OVSMOW(±1σ, ‰) 21.88±0.31 22.04±0.15 21.91±0.17 21.97±0.14 表 5 不同pH调节方法对NBS 120c PO43-回收率和δ18OPO4的影响
Table 5. Average PO43- yields and δ18OPO4values of NBS 120c under different pH adjustment methods
2 mol/L KOH 2 mol/L氨水 回收率(±1σ, n=6, %) 91.0±1.7 93.4±2.2 δ18OVSMOW(±1σ, n=6, ‰) 21.70±0.21 21.91±0.17 表 6 不同结晶温度和时间对NBS 120c PO43-回收率的影响
Table 6. Average PO43- yields of NBS 120c over different precipitation temperatures and times
温度 回收率(±1σ,n=3,%) 12 h 24 h 48 h 1周 20 ℃(室温) 27.6±4.9 79.9±2.4 91.8±0.9 (93.1±2.2)% 50 ℃ 55.0±3.3 93.6±2.1 92.7±1.9 - 表 7 实际磷灰石样品的PO43-回收率和δ18OPO4值
Table 7. The average PO43- yields and δ18OPO4 values of practical samples
样品名称 样品来源 回收率(±1σ, n=5, %) δ18OVSMOW(±1σ, n=5, ‰) DCX1 大唇犀1 95.6±2.5 10.14±0.19 DCX2 大唇犀2 96.4±1.9 10.08±0.18 SZM 三趾马 89.3±2.7 9.18±0.13 LY 羚羊 82.5±3.0 9.62±0.18 -
Amiot, R., Wang, X., Zhou, Z., et al., 2015.Environment and Ecology of East Asian Dinosaurs during the Early Cretaceous Inferred from Stable Oxygen and Carbon Isotopes in Apatite.Journal of Asian Earth Sciences, 98:358-370.doi: 10.1016/j.jseaes.2014.11.032 Chen, J., Shen, S.Z., Li, X.H., et al., 2016.High-Resolution SIMS Oxygen Isotope Analysis on Conodont Apatite from South China and Implications for the End-Permian Mass Extinction.Palaeogeography, Palaeoclimatology, Palaeoecology, 448(448):26-38.doi: 10.1016/j.palaeo.2015.11.025 Elrick, M., Witzke, B., 2016.Orbital-Scale Glacio-Eustasy in the Middle Devonian Detected Using Oxygen Isotopes of Conodont Apatite:Implications for Long-Term Greenhouse-Icehouse Climatic Transitions.Palaeogeography, Palaeoclimatology, Palaeoecology, 445:50-59.doi: 10.1016/j.palaeo.2015.12.019 Gehler, A., Gingerich, P.D., Pack, A., 2016.Temperature and Atmospheric CO2 Concentration Estimates through the PETM Using Triple Oxygen Isotope Analysis of Mammalian Bioapatite.Proceedings of the National Academy of Sciences, 113(28):7739-7744.doi: 10.1073/pnas.1518116113 Griffin, J.M., Montanez, I.P., Matthews, J.A., 2015.A Refined Protocol for δ18OPO4 Analysis of Conodont Bioapatite.Chemical Geology, 417:11-20.doi: 10.1016/j.chemgeo.2015.08.025 Halas, S., Skrzypek, G., Meier-Augenstein, W., et al., 2011.Inter-Laboratory Calibration of New Silver Orthophosphate Comparison Materials for the Stable Oxygen Isotope Analysis of Phosphates.Rapid Communications in Mass Spectrometry Rcm., 25(5):579-584.doi: 10.1002/rcm.4892 Joachimski, M.M., Breisig, S., Buggisch, W., et al., 2009.Devonian Climate and Reef Evolution:Insights from Oxygen Isotopes in Apatite.Earth & Planetary Science Letters, 284(3-4):599-609.doi: 10.1016/j.epsl.2009.05.028 LaPorte, D.F., Holmden, C., Patterson, W.P., 2009.Oxygen Isotope Analysis of Phosphate:Improved Precision Using TC/EA CF-IRMS+.Journal of Mass Spectrometry, 44(6):879-890.doi: 10.1002/jms.1549 O'Neil, J.R., Vennemann, T.W., Mckenzie, W.F., 2003.Effects of Speciation on Equilibrium Fractionations and Rates of Oxygen Isotope Exchange between (PO4) aq, and H2O.Geochimica et Cosmochimica Acta, 67(17):3135-3144.oi:10.1016/s0016-7037(02)00970-5 doi: 10.1016/S0016-7037(02)00970-5 Pucéat, E., Joachimski, M.M., Bouilloux, A., et al., 2010.Revised Phosphate-Water Fractionation Equation Reassessing Paleotemperatures Derived from Biogenic Apatite.Earth & Planetary Science Letters, 298(1-2):135-142.doi: 10.1016/j.epsl.2010.07.034 Qiao, P.J., Zhu, W.L., Shao, L., et al., 2015.Carbonate Stable Isotope Stratigraphy of Well Xike-1, Xisha Islands.Earth Science, 40(4):725-732 (in Chinese with English abstract). http://d.old.wanfangdata.com.cn/Periodical/dqkx201504014 Quinton, P.C., Macleod, K.G., 2014.Oxygen Isotopes from Conodont Apatite of the Midcontinent, US:Implications for Late Ordovician Climate Evolution.Palaeogeography, Palaeoclimatology, Palaeoecology, 404(3):57-66.doi: 10.1016/j.palaeo.2014.03.036 Rosenau, N.A., Tabor, N.J., Herrmann, A.D., 2014.Assessing the Paleoenvironmental Significance of Middle-Late Pennsylvanian Conodont Apatite δ18O Values in the Illinois Basin.Palaios, 29(6):250-265.doi: 10.2110/palo.2013.112 Sun, Y., Joachimski, M.M., Wignall, P.B., et al., 2012.Lethally Hot Temperatures during the Early Triassic Greenhouse.Science, 338(6105):366-370.doi: 10.1126/science.1224126 Sun, Y.D., Wignall, P.B., Joachimski, M.M., et al., 2016a.Climate Warming, Euxinia and Carbon Isotope Perturbations during the Carnian (Triassic) Crisis in South China.Earth & Planetary Science Letters, 444:88-100.doi: 10.1016/j.epsl.2016.03.037 Sun, Y.D., Wiedenbeck, M., Joachimski, M.M., 2016b.Chemical and Oxygen Isotope Composition of Gem-Quality Apatites:Implications for Oxygen Isotope Reference Materials for Secondary Ion Mass Spectrometry (SIMS).Chemical Geology, 440:164-178.doi: 10.1016/j.chemgeo.2016.07.013 Vennemann, T.W., Fricke, H.C., Blake, R.E., 2002.Oxygen Isotope Analysis of Phosphates:A Comparison of Techniques for Analysis of Ag3PO4.Chemical Geology, 185(3-4):321-336.doi: 10.1016/s0009-2541(01)00413-2 Wang, R., Chen, J.B., Zhao, L.S., et al., 2013.In Situ Oxygen Isotope Analysis of Conodonts by SIMS and Its Application for Paleo-Sea Surface Temperature.Global Geology, 32(4):652-658(in Chinese with English abstract). http://adsabs.harvard.edu/abs/2013agufm.v32c..05z Yang, K.H., Yu, X.G., Chu, F.Y., et al., 2016.Environmental Changes in Methane Seeps Recorded by Carbon and Oxygen Isotopes in the Northern South China Sea.Earth Science, 41(9):1206-1215(in Chinese with English abstract). http://www.en.cnki.com.cn/Article_en/CJFDTotal-DQKX201607010.htm Zhang, H., Wang, J.N., Zhu, Y.G., et al., 2015.Research and Application of Analytical Technique on δ18Op of Inorganic Phosphate in Soil.Chinese Journal of Analytical Chemistry, 43(2):187-192(in Chinese with English abstract). doi: 10.1016/S1872-2040(15)60806-4 Zhou, L.Q., Ian, S., Liu, J.H., et al., 2012.Methodology of SHRIMP In-Situ O Isotope Analysis on Conodont.Acta Geologica Sinica, 86(4):611-618(in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-DZXE201204007.htm 乔培军, 朱伟林, 邵磊, 等, 2015.西沙群岛西科Ⅰ井碳酸盐岩稳定同位素地层学.地球科学, 40(4):725-732. http://www.earth-science.net/WebPage/Article.aspx?id=3068 王润, 陈剑波, 赵来时, 等, 2013.二次离子质谱微区原位牙形石氧同位素分析及其在古海表水温记录中的应用.世界地质, 32(4):652-658. doi: 10.3969/j.issn.1004-5589.2013.04.002 杨克红, 于晓果, 初凤友, 等, 2016.南海北部甲烷渗漏系统环境变化的碳、氧同位素记录.地球科学, 41(7):1206-1215. http://www.earth-science.net/WebPage/Article.aspx?id=3329 张晗, 王佳妮, 朱永官, 等, 2015.土壤无机磷酸盐中氧同位素分析方法的研究及应用.分析化学, 43(2):187-192. http://d.old.wanfangdata.com.cn/Periodical/fxhx201502005 周丽芹, Ian, S., 刘建辉, 等, 2012.牙形石SHRIMP微区原位氧同位素分析方法.地质学报, 86(4):611-618. doi: 10.3969/j.issn.0001-5717.2012.04.007 -










 下载:
下载: