Hydrochemical Evolution Processes of Karst Groundwater in Guiyang City: Evidences from Hydrochemistry and 87Sr/86Sr Ratios
-
摘要: 岩溶地下水的水化学特征及其水岩作用过程研究对岩溶地下水合理开发利用和污染防治具有重要意义.综合利用水化学分析、主要离子比值、锶含量和87Sr/86Sr比值分析和反向水文地球化学模拟,深入分析了贵阳市地下水和地表水不同季节的水化学特征变化和水文地球化学演化过程.水化学特征分析表明,贵阳市地下水以HCO3·SO4-Ca型和HCO3-Ca·Mg型为主,水化学组成在季节和空间分布上存在一定的规律性变化,地表水与地下水的直接混合对地下水化学组成有一定的影响.锶同位素比值和水文地球化学反向模拟表明,地下水水化学组分主要受岩石风化作用的控制,以方解石和白云石为主的碳酸盐的溶解-沉淀作用以及硫酸盐和岩盐的溶解是控制研究区地下水水化学特征的重要过程,并受上覆孔隙含水层硅酸盐矿物水解的影响.Abstract: Study on hydrochemical characteristics and water-rock interaction is of great significance to ascertain the causes of groundwater pollution and the sustainable use of karst water. Hydrochemical components and types, ion ratios, strontium content, 87Sr/86Sr ratio and inverse hydrogeochemical modelling were employed to identify hydrochemical evolutional processes of karst groundwater in Guiyang City. The results show that the hydrochemical types of groundwater are mainly HCO3·SO4-Ca and HCO3-Ca·Mg, and the hydrochemical compositions of groundwater varied with time and space due to the dissolution/precipitation of different minerals. The mixing of surface water and groundwater has a certain influence on the hydrochemical characteristics of groundwater. Strontium isotope analysis and inverse hydrogeochemical modeling indicate that the hydrochemical characteristics of groundwater are mainly controlled by rock weathering. The dissolution-precipitation of carbonate dominated by calcite and dolomite as well as the dissolution of sulfate and halite are important processes to control the hydrochemical characteristics of groundwater in the study area, and are also affected by the hydrolysis of silicate minerals in the overlying porous aquifer.
-
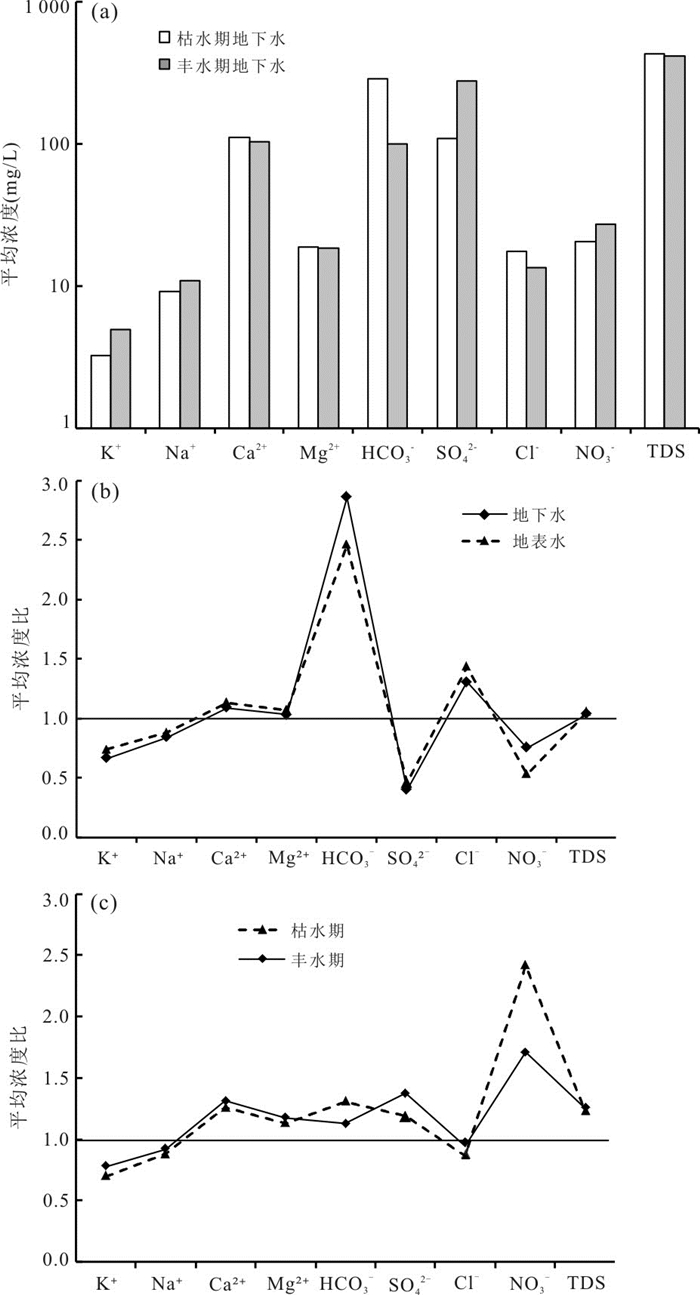
图 2 枯水期和丰水期地下水中各组分的平均浓度(a)、枯水期与丰水期不同溶质平均浓度比(b)以及地下水与地表水中不同溶质平均浓度比(c)
Fig. 2. The average concentration of different solutes in groundwater samples during the rainy and dry seasons (a), ratios of the average solute concentration for rainy season to dry seasons (b), and ratios of the average solute concentration for groundwater to surface water samples (c)
表 1 地下水TDS与离子含量相关性矩阵
Table 1. Correlation matrices of hydrochemical parameters of groundwater
K+ Na+ Ca2+ Mg2+ Cl- SO42- HCO3- TDS 枯水期
(n=41)K+ 1 Na+ 0.766 1 Ca2+ 0.108 0.277 1 Mg2+ 0.146 0.345 -0.329 1 Cl- 0.570 0.910 0.442 0.347 1 SO42- 0.375 0.635 0.573 0.343 0.791 1 HCO3- 0.153 0.374 0.150 0.651 0.361 0.269 1 TDS 0.382 0.707 0.651 0.415 0.842 0.928 0.547 1 丰水期
(n=34)K+ 1 Na+ 0.650 1 Ca2+ 0.267 0.511 1 Mg2+ 0.227 0.346 -0.174 1 Cl- 0.535 0.958 0.564 0.322 1 SO42- 0.559 0.776 0.580 0.423 0.762 1 HCO3- 0.138 0.267 0.438 0.508 0.283 0.202 1 TDS 0.539 0.832 0.764 0.431 0.847 0.890 0.552 1 表 2 研究区地下水及地表水锶含量、87Sr/86Sr及不同来源贡献率
Table 2. Statistical results of Sr2+ concentration and 87Sr/86Sr ratios of groundwater and surface water in the study area and contribution rate of different rock sources
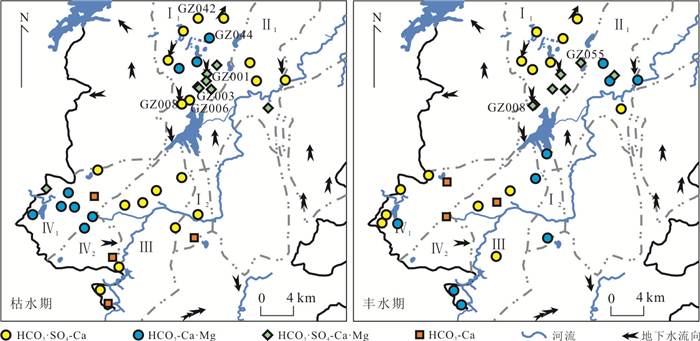
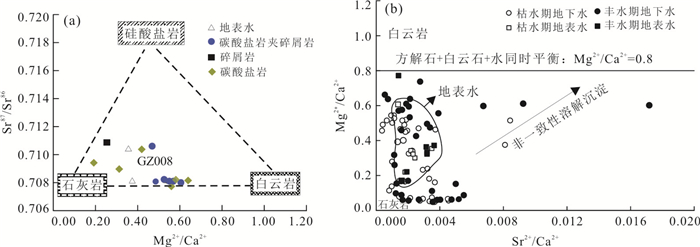
样品 类型 Sr2+(mg/L) 87Sr /86Sr 不同来源贡献率(%) 硅酸盐岩 石灰岩 白云岩 GZ075 碳酸盐岩 1.00 0.707 8 0.0 50.0 50.0 GZ004 0.55 0.708 2 0.0 48.0 52.0 GZ046 0.34 0.708 1 0.0 44.0 56.0 GZ021 0.94 0.707 7 2.0 98.0 0.0 GZ015 0.44 0.709 2 14.0 86.0 0.0 GZ054 0.34 0.708 9 9.0 74.0 17.0 GZ053 0.52 0.708 0 3.0 92.0 5.0 GZ008 0.80 0.710 3 19.0 60.0 21.0 GZ003 碳酸盐岩
夹碎屑岩0.58 0.708 2 0.0 55.0 45.0 GZ001 1.02 0.708 1 0.0 52.0 48.0 GZ047 1.59 0.708 0 0.0 46.0 54.0 GZ044 0.30 0.708 1 0.0 50.0 50.0 GZ043 0.59 0.710 6 22.0 53.0 25.0 GZ052 0.78 0.708 1 0.0 59.0 41.0 GZ055 碎屑岩 0.05 0.710 7 26.0 74.0 0.0 GZ005 地表水 0.63 0.708 0 0.0 72.0 28.0 GZ108 0.49 0.710 3 21.0 65.0 14.0 表 3 模拟地下水流路径的水样分析统计(mg/L)
Table 3. Hydrochemical data of groundwater samples along the flow path
水样 pH值 Na++K+ Ca2+ Mg2+ Cl- SO42- HCO3- 路径1 GZ044 7.07 8.6 91.8 25.3 13.6 50.6 298.0 GZ044→GZ001 GZ001 7.16 16.9 118.4 32.5 27.2 170.8 355.7 GZ001→GZ003 GZ003 6.69 24.5 123.1 33.3 39.2 203.9 342.9 路径2 GZ042 7.21 16.0 101.0 19.4 17.7 89.4 349.3 GZ042→GZ006 GZ006 6.84 29.2 116.9 25.9 31.8 145.4 334.9 表 4 反向模拟结果
Table 4. Results of the inverse hydrogeochemical modeling
路径 矿物相 转化量(mol) 化学式 GZ044→GZ001 GZ001→GZ003 路径1 方解石 -5.017×10-4 -3.095×10-4 CaCO3 白云石 3.391×10-4 3.301×10-5 CaMg(CO3)2 石膏 1.120×10-3 3.846×10-4 CaSO4·2H2O 岩盐 3.697×10-4 2.961×10-4 NaCl CO2 (g) 1.169×10-3 3.345×10-5 CO2 路径2 方解石 -9.618×10-4 CaCO3 白云石 2.677×10-4 CaMg(CO3)2 石膏 6.218×10-4 CaSO4·2H2O 岩盐 3.969×10-4 NaCl CO2 (g) 1.904×10-4 CO2 CaX2 4.378×10-4 CaX2 NaX -8.756×10-4 NaX -
Capaccioni, B., Didero, M., Paletta, C., et al., 2001. Hydrogeochemistry of Groundwaters from Carbonate Formations with Basal Gypsiferous Layers: An Example from the Mt Catria-Mt Nerone Ridge (Northern Appennines, Italy). Journal of Hydrology, 253(1-4): 14-26. https://doi.org/10.1016/s0022-1694(01)00480-2 Dang, S., 2015. Regulation of Karst Groundwater Utilization Risk and Resources Present Situation of Guiyang (Dissertation). Guizhou University, Guiyang (in Chinese with English abstract). Ding, Z.Y., Sun, N., Sun, Y.H., et al., 2015. Karst Groundwater Vulnerability and Pollution Risk Control in Guiyang City. Environmental Protection Science, 41(6): 104-112 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjbhkx201506023 Dogramaci, S. S., Herczeg, A. L., 2002. Strontium and Carbon Isotope Constraints on Carbonate-Solution Interactions and Inter-Aquifer Mixing in Groundwaters of the Semi-Arid Murray Basin, Australia. Journal of Hydrology, 262(1-4): 50-67. https://doi.org/10.1016/s0022-1694(02)00021-5 Galy, A., France-Lanord, C., Derry, L. A., 1999. The Strontium Isotopic Budget of Himalayan Rivers in Nepal and Bangladesh. Geochimica et Cosmochimica Acta, 63(13-14): 1905-1925. https://doi.org/10.1016/s0016-7037(99)00081-2 Jiang, Y.J., Yuan, D.X., 2014.Geochemical Tracers to Characterize Effects of Urbanization on Karst Groundwater Quality from Nanshan Underground River System, SW China. Quaternary Sciences, 34(5):1044-1053 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dsjyj201405013 Klaus, J. S., Hansen, B. T., Buapeng, S., 2007. 87Sr/86Sr Ratio: A Natural Tracer to Monitor Groundwater Flow Paths during Artificial Recharge in the Bangkok Area, Thailand. Hydrogeology Journal, 15(4): 745-758. https://doi.org/10.1007/s10040-007-0175-z Lang, Y.C., 2005. Geochemical Characteristics of Cycling of Substances in Karstic Groundwater System: A Case Study from Guiyang and Zunyi Cities, China (Dissertation). Institute of Geochemistry, Chinese Academy of Sciences, Guiyang (in Chinese with English abstract). Lang, Y.C., Liu, C.Q., Han, G.L., et al., 2005.Characterization of Water-Rock Interaction and Pollution of Karstic Hydrological System: A Study on Water Chemistry and Sr Isotope of Surface/Ground Water of the Guiyang Area. Quaternary Sciences, 25(5):655-662 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-DSJJ200505014.htm Li, C.S., Wu, X.C., Sun, B., et al., 2018. Hydrochemical Characteristics and Formation Mechanism of Geothermal Water in Northern Ji'nan. Earth Science, 43(S1):313-325 (in Chinese with English abstract). http://d.old.wanfangdata.com.cn/Periodical/dqkx2018z1027 Li, H., Wen, Z., Xie, X.J., et al., 2017. Hydrochemical Characteristics and Evolution of Karst Groundwater in Sanqiao District of Guiyang City. Earth Science, 42(5):804-812 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dqkx201705016 Liu, W.J., Yuan, X.M., Zhang, Y., et al., 2018. Hydrochemical Characteristics and Evolution of Karst Groundwater in Guiyang City. Geological Science and Technology Information, 37(6):245-251 (in Chinese with English abstract). http://www.en.cnki.com.cn/Article_en/CJFDTotal-DZKQ201806031.htm Lü, Y.X., Hu, W., Yang, Y., et al., 2019. Research Progress of Hydrological Cycle in Karst Critical Zone. Advances in Water Science, 30(1):123-138 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=skxjz201901013 Ma, Y.H., 2017. Process of Hydrochemical Evolution and Contamination of Karst Groundwater Systems in Guiyang City, Southwest China (Dissertation). China University of Geosciences, Wuhan (in Chinese with English abstract). Moral, F., Cruz-Sanjulián, J. J., Olías, M., 2008. Geochemical Evolution of Groundwater in the Carbonate Aquifers of Sierra de Segura (Betic Cordillera, Southern Spain). Journal of Hydrology, 360(1-4): 281-296. https://doi.org/10.1016/j.jhydrol.2008.07.012 Pu, J. B., Yuan, D. X., Zhang, C., et al., 2012. Identifying the Sources of Solutes in Karst Groundwater in Chongqing, China: A Combined Sulfate and Strontium Isotope Approach. Acta Geologica Sinica (English Edition), 86(4): 980-992. https://doi.org/10.1111/j.1755-6724.2012.00722.x Wang, J.Y., Wang, J.L., Jin, M.G., 2017. Hydrochemical Characteristics and Formation Causes of Karst Water in Jinan Spring Catchment. Earth Science, 42(5): 821-831 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dqkx201705018 Wang, Y. X., Guo, Q. H., Su, C. L., et al., 2006. Strontium Isotope Characterization and Major Ion Geochemistry of Karst Water Flow, Shentou, Northern China. Journal of Hydrology, 328(3-4): 592-603. https://doi.org/10.1016/j.jhydrol.2006.01.006 Wen, B., Zhou, J. W., Zhou, A. G., et al., 2016. Sources, Migration and Transformation of Antimony Contamination in the Water Environment of Xikuangshan, China: Evidence from Geochemical and Stable Isotope (S, Sr) Signatures. Science of the Total Environment, 569-570: 114-122. https://doi.org/10.1016/j.scitotenv.2016.05.124 Xu, S., Li, S.L., Zhong, J., et al., 2018. Hydrochemical Characteristics and Chemical Weathering Processes in Chishui River Basin. Chinese Journal of Ecology, 37(3):667-678 (in Chinese with English abstract). http://d.old.wanfangdata.com.cn/Periodical/stxzz201803007 Yang, X.L., Zeng, Q., Su, Z.Z., et al., 2010. Pollution Condition and Prevention of Groundwater Pollution in Guiyang City. Guizhou Geology, 27(4):291-295 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gzdz201004012 Zha, X.F., Wu, P., Zhu, L.J., et al., 2008. Sustainable Development and Utilization and Measures of Karst Groundwater in Guiyang City. Environmental Protection and Technology, 14(1):27-30 (in Chinese with English abstract). Zhai, Y.Z., Wang, J.S., Zuo, R., et al., 2011. Strontium Isotopic Tracing of Water-Rock Interaction in Quaternary Aquifer in Beijing Plain. Science & Technology Review, 29(6):17-20 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kjdb201106006 Zhao, J.T., Zhou, J.L., Gao, Y.X., et al., 2016.Spatial- Temporal Evolution of Total Dissolved Solids of Groundwater in Plain Area of Yanqi Basin, Xinjiang. Transactions of the Chinese Society of Agricultural Engineering, 32(5):120-125 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=nygcxb201605017 党爽, 2015.贵阳地区岩溶地下水资源现状及开发利用风险评价(硕士学位论文).贵阳: 贵州大学. http://cdmd.cnki.com.cn/Article/CDMD-10657-1015910972.htm 丁贞玉, 孙宁, 孙运海, 等, 2015.贵阳市岩溶地下水污染风险与防控监管.环境保护科学, 41(6): 104-112. doi: 10.3969/j.issn.1004-6216.2015.06.023 蒋勇军, 袁道先, 2014.城市发展对岩溶地下水质影响的地球化学示踪——以重庆南山老龙洞地下河系统为例.第四纪研究, 34(5):1044-1053. http://d.old.wanfangdata.com.cn/Conference/8868898 郎赟超, 2005.喀斯特地下水文系统物质循环的地球化学特征——以贵阳市和遵义市为例(博士学位论文).贵阳: 中国科学院地球化学研究所. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y856107 郎赟超, 刘丛强, 韩贵琳, 等, 2005.贵阳市区地表/地下水化学与锶同位素研究.第四纪研究, 25(5):655-662. doi: 10.3321/j.issn:1001-7410.2005.05.015 李常锁, 武显仓, 孙斌, 等, 2018.济南北部地热水水化学特征及其形成机理.地球科学, 43(S1):313-325. http://d.old.wanfangdata.com.cn/Periodical/dqkx2018z1027 李华, 文章, 谢先军, 等, 2017.贵阳市三桥地区岩溶地下水水化学特征及其演化规律.地球科学, 42(5):804-812. http://d.old.wanfangdata.com.cn/Periodical/dqkx201705016 刘伟江, 袁祥美, 张雅, 等, 2018.贵阳市岩溶地下水水化学特征及演化过程分析.地质科技情报, 37(6):245-251. http://d.old.wanfangdata.com.cn/Periodical/dzkjqb201806031 吕玉香, 胡伟, 杨琰, 等, 2019.岩溶关键带水循环过程研究进展.水科学进展, 30(1):123-138. http://d.old.wanfangdata.com.cn/Periodical/skxjz201901013 马燕华, 2017.西南岩溶地区地下水系统水化学演化过程及污染成因研究——以贵阳市为例(硕士学位论文).武汉: 中国地质大学. 王珺瑜, 王家乐, 靳孟贵, 2017.济南泉域岩溶水水化学特征及其成因.地球科学, 42(5): 821-831. http://d.old.wanfangdata.com.cn/Periodical/dqkx201705018 徐森, 李思亮, 钟君, 等, 2018.赤水河流域水化学特征与岩石风化机制.生态学杂志, 37(3):667-678. http://d.old.wanfangdata.com.cn/Periodical/stxzz201803007 杨秀丽, 曾群, 苏泽志, 等, 2010.贵阳市地下水污染现状评价及防治对策.贵州地质, 27(4):291-295. doi: 10.3969/j.issn.1000-5943.2010.04.012 查学芳, 吴攀, 朱立军, 等, 2008.贵阳市岩溶地下水可持续开发利用与对策.环保科技, 14(1):27-30. doi: 10.3969/j.issn.1674-0254.2008.01.006 翟远征, 王金生, 左锐, 等, 2011.北京平原区第四系含水层中水-岩作用的锶同位素示踪.科技导报, 29(6):17-20. doi: 10.3981/j.issn.1000-7857.2011.06.001 赵江涛, 周金龙, 高业新, 等, 2016.新疆焉耆盆地平原区地下水溶解性总固体时空演化.农业工程学报, 32(5):120-125. http://d.old.wanfangdata.com.cn/Periodical/nygcxb201605017 -









 下载:
下载: