Formation Mechanism and Geological Construction Constraints of Metasilicate Mineral Water in Yudaokou, Hannuoba Basalt Area
-
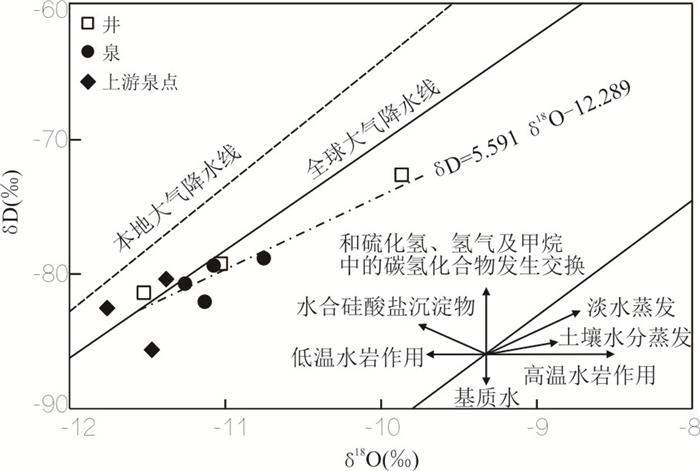
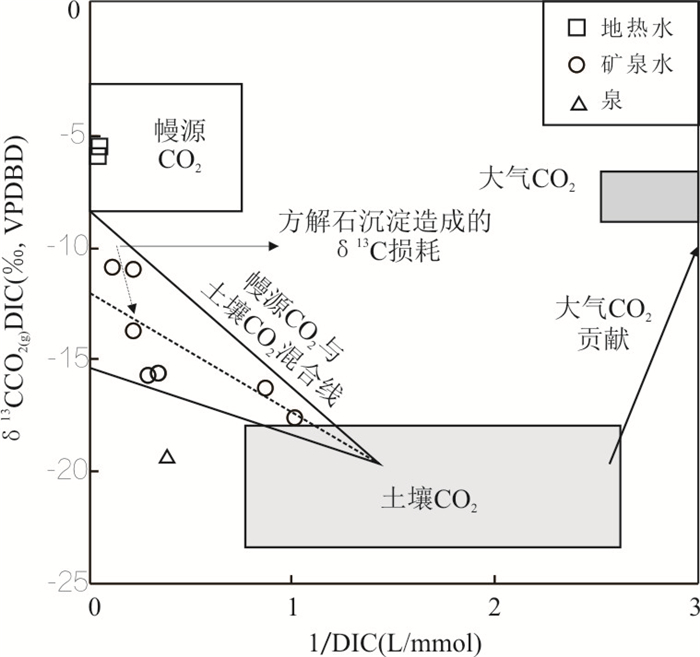
摘要: 冀北坝上一带玄武岩地区广布富偏硅酸地下水,研究其形成机制及其水岩作用过程对矿泉水的合理开发利用与京津冀水源涵养功能具有重要意义.结合玄武岩地质建造地下水赋存特征,综合利用水化学分析,玄武岩岩石风化机制,水岩相互作用矿物平衡体系,δD、δ18O和δ13C同位素、14C放射性同位素测年等方法,剖析了汉诺坝玄武岩偏硅酸矿泉水形成的岩石地球化学风化和水文地球化学过程及地质建造制约因素.结果表明,研究区矿泉水为低矿化度的HCO3-Ca·Mg型与HCO3-Na·Ca型水,矿泉水形成类型有构造断裂深循环淋溶型和层状补给富集埋藏型2类.上层古风化壳地下水14C校正年龄约为4 050 a,地下水可溶性无机碳来源于土壤CO2与幔源CO2的混合作用.偏硅酸矿泉水的形成与分布受玄武岩地质建造制约,受岩石地球化学特征、岩石风化地表过程和水文地球化学响应过程控制.地下水中偏硅酸主要来源于玄武岩中斜长石、单斜辉石、镁橄榄石等硅酸盐矿物的风化水解;岩石矿物风化的水化学响应过程受溶滤作用控制,受阳离子交换作用影响.Abstract: Metasilicate mineral groundwater is widely distributed in basaltic area of Bashang area in North Hebei Province. It is of great significance to ascertain the formation mechanism and water-rock interaction process for the rational development and utilization of mineral water and the water conservation function of Beijing-Tianjin-Hebei region. Hydrochemical components statistical analysis, chemical weathering process of basaltic, mineral equilibrium phase of water-rock interaction process, δD, δ18O, δ13C isotopes and radioisotope dating by 14C were used to identify the geological construction constraints and ascertain the formation mechanism of metasilicate mineral groundwater. The results show that the mineral groundwater of study area is characterized by low mineralization while the main hydrochemical types of groundwater are HCO3- Ca·Mg and HCO3- Na·Ca. The outcropping mechanism of mineral water can be divided into two types:deep cyclic leaching of tectonic faults and stratified enrichment of recharge type. The age of groundwater in the upper paleoweathering crust is about 4 050 a, and the dissolved inorganic carbon of groundwater comes from the mixing of soil CO2 and mantle CO2. Metasilicic acid in groundwater originates from the weathering and hydrolysis of plagioclase, pyroxene and forsterite in basaltic. Hydrogeochemical response of rock weathering is controlled by rock mineral leaching and affected by hydrochemical parameters cation exchange. The formation and distribution of metasilicate mineral water are restricted by the basalt geological construction, and controlled by weathering mechanism of rocks and hydrogeochemical conditions.
-
Key words:
- metasilicate mineral water /
- basalt /
- hydrogeochemistry /
- weathering mechanism /
- geological construction /
- Yudaokou /
- geochemistry
-
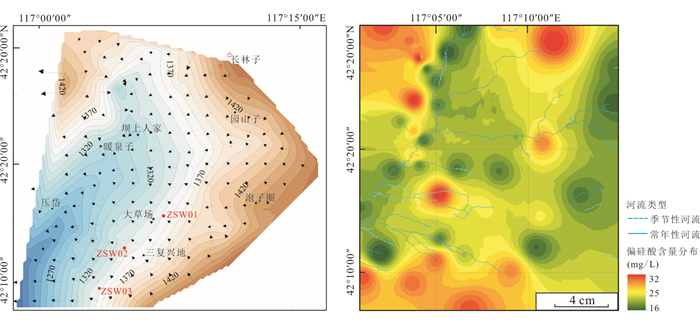
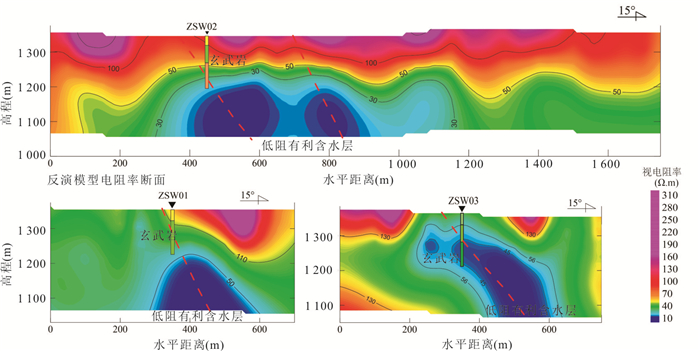
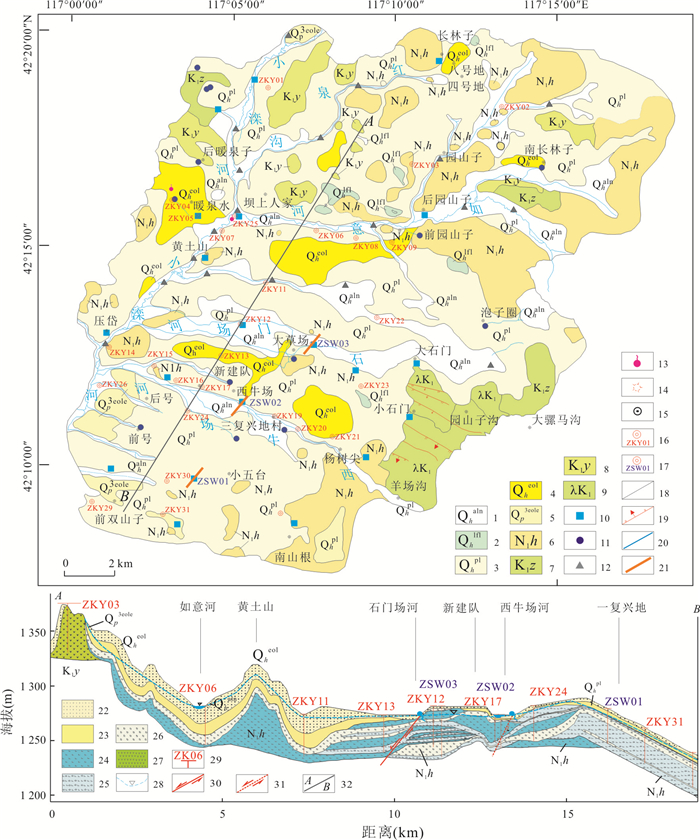
图 1 研究区地质图与水质样品分布
1.冲洪积砂砾石,亚砂土;2.湖沼积亚粘土,淤泥;3.洪积亚砂土、细砂;4.风积粉细砂;5.风积残积亚粘土,粉砂夹残积碎石;6.汉诺坝玄武岩,致密块状橄榄玄武岩,气孔状橄榄玄武岩、橄榄辉石玄武岩及安山质玄武岩;7.张家口组角砾凝灰岩,流纹质凝灰岩,凝灰质砂砾岩,流纹岩;8.义县组安山岩,气孔杏仁状安山岩;9.潜流纹岩;10.井水样品;11.泉样品;12.地表水样品;13.地热水样品;14.火山口;15.村庄;16.工程地质钻孔;17.水文地质钻孔;18.地质界线;19.正断层;20.河流水系;21.物探剖面;22.风积物;23.冲洪积-湖沼积物;24.橄榄玄武岩;25.气孔橄榄玄武岩;26.古风化壳;27.安山岩、粗安岩;28.地下水位;29.钻孔;30.正断层;31.推测正断层;32.剖面位置
Fig. 1. Geological sketch map and the sampling sites of study area
图 4 玄武岩典型剖面与岩石标本及镜下特征显微照片
a.玄武喷溢-风化旋回典型剖面;b.炉渣状玄武岩孔洞与玄武岩次生孔洞;c.玄武岩风化“土包石”结构;d.钻孔古风化壳地层;e.蚀变气孔-杏仁状玄武岩;f.气孔内柱状结晶矿物;g.蜂窝状气孔橄榄玄武岩;h.麻状气孔玄武岩;i.针状气孔玄武岩;j.杏仁状橄榄玄武岩;k.致密橄榄玄武岩;l.致密橄榄玄武岩薄片;m.致密橄榄玄武岩腐岩壳薄片;n.蜂窝状气孔状橄榄玄武岩薄片;o.针状气孔玄武岩薄片;Pl.斜长石;Ol.橄榄石;Cpx.单斜辉石;Mt.磁铁矿
Fig. 4. Typical basalt sections, basalt specimens and microtextures of basalt samples
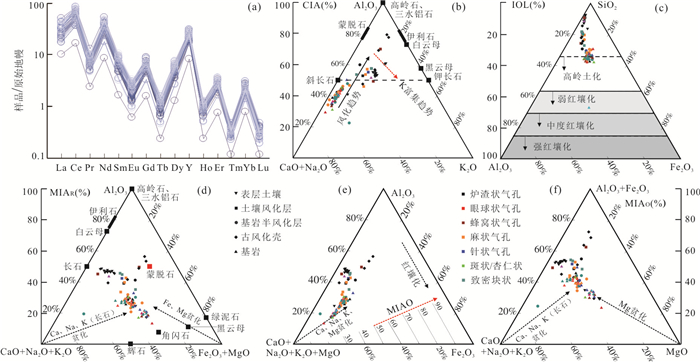
图 6 玄武岩及其风化物稀土配分曲线与岩(土)CIA、IOL、MIA风化指数三元图
a.玄武岩及其风化物稀土配分曲线;b.Al2O3-CaO+Na2O-K2O (A-CN-K)化学蚀变指数(CIA)图;c.SiO2-Al2O3-Fe2O3 (SAF)红土化指数(IOL); d.A-CNK-FM还原镁铁质蚀变指数(MIAR); e.A-L-F氧化镁铁质蚀变指数(MIAo);f.AF-CNK-M氧化镁铁质蚀变指数(MIAo)
Fig. 6. The rare earth distribution curve and the CIA, IOL and MIA weathering indexes of basalt rocks (soil)
表 1 研究区水化学参数统计
Table 1. Statistics of hydrochemical parameters of study area
类别 项目 TDS K+ Na+ Ca2+ Mg2+ HCO3- SO42- Cl- SiO2 TFe 游离CO2 pH 地下水 Min 122.00 0.52 6.96 1.65 0.78 74.77 0.05 2.31 10.90 0.01 ND 6.44 Max 795.57 19.58 73.29 128.20 53.32 263.00 90.95 61.09 31.93 37.54 84.66 9.24 Mean 303.81 3.16 19.24 38.84 14.40 141.50 18.86 15.31 23.78 3.15 26.70 7.52 Std. 169.78 3.98 14.98 29.86 11.43 53.19 23.98 18.14 5.23 8.01 26.17 0.60 Cv 0.56 1.26 0.78 0.77 0.79 0.38 1.27 1.18 0.22 2.54 0.98 0.08 泉 Min 128.00 0.65 6.13 15.98 2.83 64.93 0.39 1.75 16.78 0.01 2.07 6.72 Max 244.59 3.22 59.03 37.41 13.98 152.16 23.36 6.62 30.50 25.85 65.85 7.69 Mean 189.96 1.73 13.14 24.65 8.01 103.79 10.44 3.75 23.63 2.41 38.92 7.17 Std. 38.15 0.87 13.39 6.26 3.65 31.33 6.10 1.16 4.16 6.82 16.38 0.32 Cv 0.20 0.50 1.02 0.25 0.46 0.30 0.58 0.31 0.18 2.83 0.42 0.04 地表水 Min 76.00 1.09 6.64 13.23 3.98 52.47 0.02 3.34 5.87 0.30 20.70 6.92 Max 260.00 4.99 18.56 48.67 18.93 242.67 12.99 15.11 24.83 12.25 86.55 8.78 Mean 181.12 2.28 12.55 29.68 11.29 147.23 5.31 6.06 18.75 2.10 51.55 7.58 Std. 45.37 0.82 2.84 9.23 4.57 54.66 4.68 2.86 4.62 2.57 19.10 0.38 Cv 0.25 0.36 0.23 0.31 0.41 0.37 0.88 0.47 0.25 1.23 0.37 0.05 地热水 DR01 1 804.67 2.38 453.80 1.64 0.49 1 078.00 26.03 44.55 25.86 0.19 148.00 8.26 DR02 2 559.13 2.16 682.50 2.40 0.12 1 657.76 15.93 36.64 20.00 0.22 102.00 8.23 大气降水 JS01 11.38 0.37 0.26 2.01 0.19 5.64 2.35 0.41 0.16 ND 2.07 6.40 注:Min.最小值;Max.最大值;Mean.均值;Std.标准偏差;Cv.变异系数;ND.未检出;pH无量纲,其余单位为mg/L. 表 2 研究区水化学组分相关系数矩阵
Table 2. Correlation coefficients of hydrochemical parameters of study area
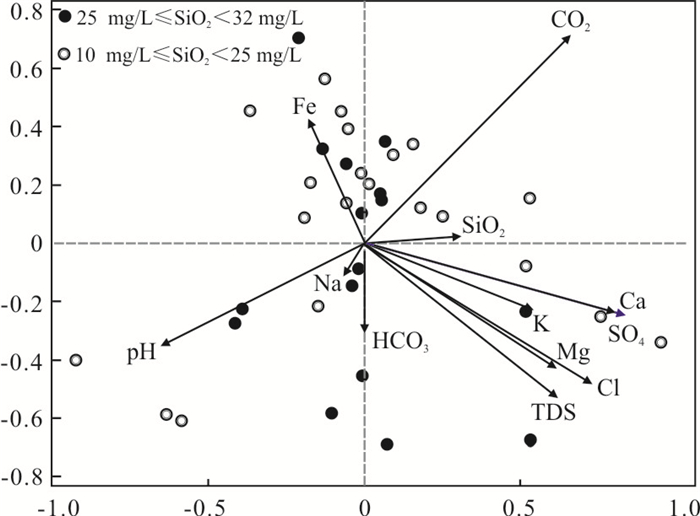
TDS K+ Na+ Ca2+ Mg2+ HCO3- SO42- Cl- SiO2 CO2 pH TFe TDS 1.000 K+ 0.261 1.000 Na+ 0.051 -0.060 1.000 Ca2+ 0.961** 0.202 -0.055 1.000 Mg2+ 0.945** 0.187 -0.078 0.933** 1.000 HCO3- 0.490 0.026 0.070 0.437 0.558 1.000 SO42- 0.790* 0.308 0.010 0.819** 0.737* -0.024 1.000 Cl- 0.858** 0.231 0.082 0.866** 0.799** 0.277 0.789** 1.000 SiO2 0.102 0.027 -0.305 0.172 0.059 -0.022 0.004 0.101 1.000 CO2 0.174 0.005 0.057 0.276 0.186 -0.270 0.476 0.323 -0.035 1.000 pH -0.223 -0.239 0.372 -0.331 -0.182 0.269 -0.475 -0.263 -0.293 -0.585 1.000 TFe -0.099 -0.085 -0.088 -0.171 -0.082 0.025 -0.166 -0.143 -0.160 0.011 -0.062 1.000 注:**表示在0.01水平(双侧)上显著相关; *表示在0.05水平(双侧)上显著相关. 表 3 矿物的溶解反应方程式
Table 3. Chemical equations of mineral dissolution
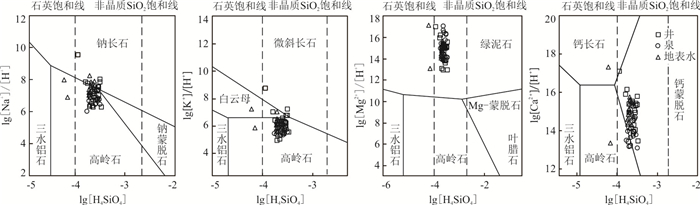
矿物 反应方程式 斜长石 Na0.62Ca0.38Al1.38Si2.62O8+1.38CO2+4.55H2O=0.69Al2Si2O5(OH)4+0.62Na++0.38Ca2++1.38HCO3-+1.24H2SiO42- 钙长石 CaA12Si2O8+2CO2+8H2O=A12O3+3H2O+Ca2++2H2SiO42-+2HCO3- 钠长石 2NaAlSi3O8+2CO2+11H2O= Al2Si2O5(OH)4+2Na++2HCO3-+4H4SiO4 辉石 [CaMg0.7Al0.6Si1.7]O6+3.4CO2+4.5H2O=0.3Al2Si2O5(OH)4+Ca2++0.7Mg2++1.1H4SiO4+ 3.4HCO3- 镁橄榄石 Mg2SiO4+4H2O=2Mg(OH)2+ H4SiO4 绿泥石 Mg5Al2Si3O10(OH)6+10H2O=5Mg2++ 2Al(OH)4-+3H4SiO4+8OH- 钙蒙脱石 6Ca0.167Al2.33Si3.67O10(OH)2+60H2O+12OH-=Ca2++14Al(OH)4-+22H4SiO4 镁蒙脱石 6Mg0.167Al2.33Si3.67O10(OH)2+60H2O+12OH-=Mg2++14Al(OH)4-+22H4SiO4 -
Alexandra, M., Niko, K., Hazel, C., et al., 2015. Kinetics of CO2-Fluid-Rock Reactions in a Basalt Aquifer, Soda Springs, Idaho. Applied Geochemistry, 61(Oct.):272-283. https://doi.org/10.1016/j.apgeochem.2015.06.010 Babechuk, M.G., Widdowson, M., Kamber, B. S., 2014. Quantifying Chemical Weathering Intensity and Trace Element Release from Two Contrasting Basalt Profiles, Deccan Traps, India. Chemical Geology, 363(Jan.):56-75. https://doi.org/10.1016/j.chemgeo.2013.10.027 Bagdavadze, L., Beon, O., Hrkal, Z., et al., 2008. Effects of Groundwater Exploitation on the Borjomi Mineral Water Reservoir in Georgia. Environmental Geology, 54(6):1301-1311. https://doi.org/10.1007/s00254-007-0913-5 Bhattacharyya, T., Pal, D. K., Srivastava, P., 1999. Role of Zeolites in Persistence of High Altitude Ferruginous Alfisols of the Humid Tropical Western Ghats, India. Geoderma, 90(3/4):263-276. https://doi.org/10.1016/s0016-7061(98)00122-0 Fan, Q.C., Du, X.X., Sui, J.L., et al., 2010. Genesis of Carbonatite from Hannuoba and Yangyuan. Acta Petrologica Sinica, 26(11):3189-3194 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-YSXB201011003.htm Floury, P., Gaillardet, J., Tallec, G., et al., 2019. Chemical Weathering and CO2 Consumption Rate in a Multilayered-Aquifer Dominated Watershed under Intensive Farming:The Orgeval Critical Zone Observatory, France. Hydrological Processes, 33(2):195-213. https://doi.org/10.1002/hyp.13340 Gaillardet, J., Dupré, B., Louvat, P., et al., 1999. Global Silicate Weathering and CO2 Consumption Rates Deduced from the Chemistry of Large Rivers. Chemical Geology, 159(1/2/3/4):3-30. https://doi.org/10.1016/s0009-2541(99)00031-5 Gao, M., Chen, Y., 1995. Study on the Interactions between Water and Basalt in a Closed System. Journal of Nanjing University (Natural Science Edition), 31(3):476-486 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-NJDZ503.017.htm Guo, Q.H., Wang, Y.X., 2014. Simulation of Geochemical Processes Affecting Groundwater in Quaternary Porous Aquifers of Taiyuan Basin:A Typical Cenozoic Rift Basin. Earth Science Frontiers, 21(4):83-90 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-DXQY201404012.htm Han, G.L., Liu, C. Q., 2005. Hydrogeochemistry of Rivers in Guizhou Province, China:Constraints on Crustal Weathering in Karst Terrain. Advances in Earth Science, 20(4):394-406 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dqkxjz200504004 Hasan, M., Shang, Y. J., Jin, W. J., 2018. Delineation of Weathered/Fracture Zones for Aquifer Potential Using an Integrated Geophysical Approach:A Case Study from South China. Journal of Applied Geophysics, 157:47-60. https://doi.org/10.1016/j.jappgeo.2018.06.017 Koh, D. C., Genereux, D. P., Koh, G. W., et al., 2017. Relationship of Groundwater Geochemistry and Flow to Volcanic Stratigraphy in Basaltic Aquifers Affected by Magmatic CO2, Jeju Island, Korea. Chemical Geology, 467:143-158. https://doi.org/10.1016/j.chemgeo.2017.08.009 Li, S., 2012. Experimental Study of the Characteristic Component(HSiO3-、Sr2+)Genesis of the Mineral Water in the Basalt in Jingyu County (Dissertation). Jilin University, Changchun (in Chinese with English abstract). Liang, X. J., Li, S., Li, Y. X., et al., 2013. Experimental Study of Evolution of Aqueous SiO2 in the Mineral Water in Basalt Beds of Jingyu County, China. Procedia Earth and Planetary Science, 7:500-503. https://doi.org/10.1016/j.proeps.2013.03.118 Liu, Q. X., Wang, G. L., Zhang, F. W., 2004. Geological-Geochemical Environment for the Enrichment of Trace Component H2SiO3 in Groundwater. Acta Geoscientica Sinica, 22(5):575-578 (in Chinese with English abstract). http://www.zhangqiaokeyan.com/academic-journal-cn_acta-geoscientica-sinica_thesis/0201253098442.html Nesbitt, H. W., Young, G. M., 1982. Early Proterozoic Climates and Plate Motions Inferred from Major Element Chemistry of Lutites. Nature, 299(5885):715-717. https://doi.org/10.1038/299715a0 Nesbitt, H. W., Young, G. M., 1984. Prediction of Some Weathering Trends of Plutonic and Volcanic Rocks Based on Thermodynamic and Kinetic Considerations. Geochimica et Cosmochimica Acta, 48(7):1523-1534. https://doi.org/10.1016/0016-7037(84)90408-3 Schoeller, H., 1967. Qualitative Evaluation of Groundwater Resource:Methods and Techniques of Groundwater Investigation and Development. Water Research, 33:44-52. http://www.researchgate.net/publication/298455180_Qualitative_evaluation_of_groundwater_resources_in_methods_and_techniques_of_groundwater_investigation_and_development Shan, T.T., Xu, S.G., Fan, Z.G., et al., 2019. Characteristics and Formation Mechanism of Metasilicate Mineral Water in Xishan Mountain of Kunming. Journal of Kunming University of Science and Technology (Natural Science Edition), 44(2):39-47 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-KMLG201902007.htm Stefánsson, A., Sveinbjörnsdóttir, Á. E., Heinemeier, J., et al., 2016. Mantle CO2 Degassing through the Icelandic Crust:Evidence from Carbon Isotopes in Groundwater. Geochimica et Cosmochimica Acta, 191:300-319. https://doi.org/10.1016/j.gca.2016.06.038 Su, C. L., Zhang, Y., Ma, Y. H., et al., 2019. Hydrochemical Evolution Processes of Karst Groundwater in Guiyang City:Evidences from Hydrochemistry and 87Sr/86Sr Ratios. Earth Science, 44(9):2829-2838 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-DQKX201909002.htm Sun, H.Y., Mao, Q.G., Wei, X.F., et al., 2018. Hydrogeochemical Characteristics and Formation Evolutionary Mechanism of the Groundwater System in the Hami Basin. Geology in China, 45(6):1128-1141 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-DIZI201806005.htm Sun, H.Y., Wei, X.F., Gan, F.W., et al., 2020. Genetic Type and Formation Mechanism of Strontium-Rich Groundwater in the Upper and Middle Reaches of Luanhe River Basin. Acta Geoscientia Sinica, 41(1):65-79 (in Chinese with English abstract). Sun, R. L., 2006. Scale Effects and Sequential Indicator Simulation of Hydraulic Conductivity in Basalt (Dissertation). China University of Geosciences, Wuhan (in Chinese with English abstract). Tan, J. W., Liu, Y. M., 1983. Formation Conditions and Hydrogeological Characteristics of Secondary Caves in Hannuoba Basalt. Journal of Hebei GEO University, 6(3):23-35 (in Chinese with English abstract). Tang, J., Xu, W. L., Li, Y., et al., 2019. Composition Variations of Mesozoic and Cenozoic Basalts in Northern Great Xing' an Range:Implications for Thermal Evolution of Mantle. Earth Science, 44(4):1096-1112 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-DQKX201904004.htm Wang, Z. B., Shen, L. F., Xu, Z.M., 2016. Hydrochemical Characteristics and Their Implication for the Water-Rock/Soil Interaction in the Touzhai Landslide. Hydrogeology & Engineering Geology, 43(1):111-116, 123 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-SWDG201601018.htm Wei, R. C., 2014. Study on the Formation Mechanism of Natural Mineral Water in Jingyu National Nature Reserve (Dissertation). Jilin University, Changchun (in Chinese with English abstract). Wu, Y., Gibson, C. E., 1996. Mechanisms Controlling the Water Chemistry of Small Lakes in Northern Ireland. Water Research, 30(1):178-182. https://doi.org/10.1016/0043-1354(95)00140-g Xu, Z. M., 2013. The Chemical Water-Rock Interaction in Silicate Rock Slopes. Acta Geologica Sinica, 87(6):860-871 (in Chinese with English abstract). http://epub.cnki.net/grid2008/docdown/docdownload.aspx?filename=DZXE201306010&dbcode=CJFD&year=2013&dflag=pdfdown Xu, Z. M., Huang, R.Q., 2013. The Assessment of the Weathering Intensity of Emeishan Basalt Based on Rock Blocks (Ⅰ):Geochemistry of Weathered Basalt Blocks. Geology in China, 40 (3):895-908 (in Chinese with English abstract). http://www.cnki.com.cn/Article/CJFDTotal-DIZI201303021.htm Yan, B. Z., Xiao, C. L., Liang, X. J., et al., 2016. Hydrogeochemical Tracing of Mineral Water in Jingyu County, Northeast China. Environmental Geochemistry and Health, 38(1):291-307. https://doi.org/10.1007/s10653-015-9719-7 Ye, H. J., Zhang, R. X., Wu, P., et al., 2019. Characteristics and Driving Factor of Hydrochemical Evolution in Karst Water in the Critical Zone of Liupanshui Mining Area. Earth Science, 44(9):2887-2898 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-DQKX201909007.htm 樊祺诚, 杜星星, 隋建立, 等, 2010.汉诺坝-阳原火成碳酸岩成因探讨.岩石学报, 26(11):3189-3194. http://qikan.cqvip.com/Qikan/Article/Detail?id=1003851223 高明, 陈芸, 1995.封闭体系中水与玄武岩作用的研究.南京大学学报(自然科学版), 31(3):476-486. http://www.cnki.com.cn/Article/CJFDTotal-NJDZ503.017.htm 郭清海, 王焰新, 2014.典型新生代断陷盆地内孔隙地下水地球化学过程及其模拟:以山西太原盆地为例.地学前缘, 21(4):83-90. http://www.cnki.com.cn/Article/CJFDTotal-DXQY201404012.htm 韩贵琳, 刘丛强, 2005.贵州喀斯特地区河流的研究‒碳酸盐岩溶解控制的水文地球化学特征.地球科学进展, 20(4):394-406. http://d.old.wanfangdata.com.cn/Periodical/dqkxjz200504004 李树, 2012.靖宇玄武岩矿泉水中特征组分(HSiO3-、Sr2+)成因的实验研究(博士学位论文).长春: 吉林大学. 刘庆宣, 王贵玲, 张发旺, 2004.地下水中微量组份H2SiO3富集的地质地球化学环境.地球学报, 22(5):575-578. http://www.cqvip.com/Main/Detail.aspx?id=11073608 单婷婷, 徐世光, 范柱国, 等, 2019.昆明西山偏硅酸矿泉水特征及形成机理.昆明理工大学学报(自然科学版), 44(2):39-47. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kmlgdxxb201902007 苏春利, 张雅, 马燕华, 等, 2019.贵阳市岩溶地下水水化学演化机制:水化学和锶同位素证据.地球科学, 44(9):2829-2838. doi: 10.3799/dqkx.2019.214 孙厚云, 毛启贵, 卫晓锋, 等, 2018.哈密盆地地下水系统水化学特征及形成演化.中国地质, 45(6):1128-1141. http://d.wanfangdata.com.cn/periodical/zgdizhi201806005 孙厚云, 卫晓锋, 甘凤伟, 等, 2020.滦河流域中上游富锶地下水成因类型与形成机制.地球学报, 41(1):65-79. http://d.wanfangdata.com.cn/periodical/dqxb202001005 孙蓉琳, 2006.玄武岩渗透系数尺度效应及顺序指示模拟(博士学位论文).武汉: 中国地质大学. 谭绩文, 刘亚民, 1983.汉诺坝玄武岩次生洞穴形成条件及其水文地质特征.河北地质学院学报, 6(3):23-35. http://www.cnki.com.cn/Article/CJFDTotal-HBDX198303003.htm 唐杰, 许文良, 李宇, 等, 2019.大兴安岭北段中-新生代玄武岩成分变异:对地幔热演化过程意义.地球科学, 44(4):1096-1112. doi: 10.3799/dqkx.2019.055 王志兵, 申林方, 徐则民, 2016.头寨滑坡地下水化学特征及其反映的水-岩(土)相互作用.水文地质工程地质, 43(1):111-116, 123. http://d.wanfangdata.com.cn/Periodical/swdzgcdz201601017 危润初, 2014.靖宇国家级自然保护区天然矿泉水形成机理研究(博士学位论文).长春: 吉林大学. 徐则民, 2013.硅酸盐岩斜坡水岩化学作用.地质学报, 87(6):860-871. http://d.wanfangdata.com.cn/Periodical/dizhixb201306010 徐则民, 黄润秋, 2013.基于结构体的峨眉山玄武岩风化程度评价(Ⅰ):风化结构体地球化学.中国地质, 40(3):895-908. http://www.cnki.com.cn/Article/CJFDTotal-DIZI201303021.htm 叶慧君, 张瑞雪, 吴攀, 等, 2019.六盘水矿区关键带岩溶水水化学演化特征及驱动因子.地球科学, 44(9):2887-2898. doi: 10.3799/dqkx.2019.201 -








 下载:
下载: