Research Status and Application Potential of CO2 Mineralization
-
摘要: CO2浓度急剧上升成为一个很严峻的问题,因此,降低大气CO2浓度成为当务之急.目前涉及的方案中的海洋封存、地质封存,虽封存潜力巨大,但带来的负面影响也不容小觑.CO2矿化利用实质是模拟自然界岩石化学风化,作为一种新兴的减排方案,既能固定大气CO2,生成具有工业附加值的碳酸盐产品,又能实现环境友好.能够矿化利用的原材料包括天然富钙、镁硅酸盐矿物,工业碱性废固、液,盐湖中的氯化镁资源等,矿化利用的方法也不尽相同.虽然硅酸盐岩的风化是如何控制长时间尺度的气候变化的机制还没有定论,但风化过程中具有固定大量CO2的潜力这一认识已达成共识.对含有大量硅酸盐矿物的尾矿矿化CO2的研究是目前的热点,介绍了尾矿矿化CO2的研究现状及几种重要尾矿矿物的矿化应用潜力.Abstract: The sharp rise of carbon dioxide concentration has become a very serious problem, so reducing the atmospheric carbon dioxide concentration has become a top priority. Although the potential of marine and geological sequestration is huge, the negative impact of these schemes can not be underestimated. As a new emission reduction scheme, carbon dioxide mineralization can not only fix atmospheric carbon dioxide, generate carbonate products with industrial added value, but also achieve environmental friendliness. Raw materials that can be used for mineralization include natural calcium-rich, magnesium silicate minerals, industrial alkaline waste solids, liquids, magnesium chloride resources in salt lakes, etc. The methods of mineralization are also different. Although the mechanism of how the weathering of silicate rocks can control the long-term climate change has not been determined, there is a consensus that the weathering process has the potential to fix a large amount of CO2. The research on the mineralized CO2 of tailing containing a lot of silicate minerals is a hot spot at present. This paper introduces the research status of mineralized CO2 of tailing and the mineralized potential of several important tailing minerals.
-
Key words:
- CO2 mineralization /
- alkaline minerals /
- industrial waste /
- tailings /
- carbonation reaction /
- mineral deposite
-
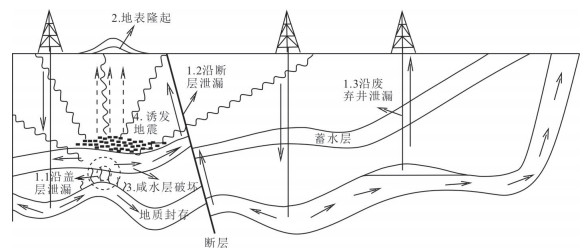
图 1 CO2地质封存方式的泄漏风险图据(谢和平等,2012修改)
Fig. 1. Leakage risk map for CO2 geological storage(modified from Xie et al., 2012)
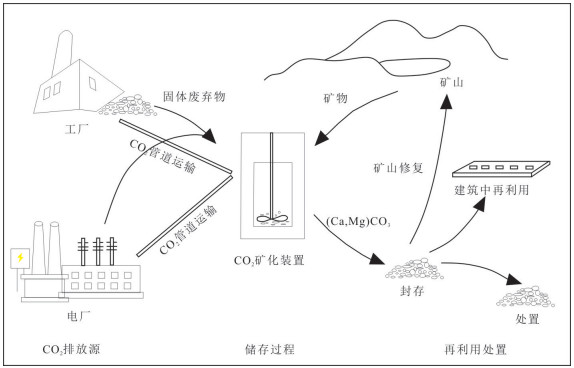
图 2 CO2矿化封存示意图(Metz et al., 2005)
Fig. 2. Schematic of a CO2 mineral carbonation operation(modified from Metz et al., 2005)
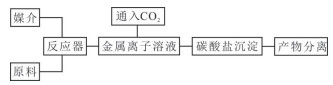
表 1 用于矿化的工业废固、液主要成分及其反应方程式
Table 1. Main compositions of industrial waste solids and liquids for mineralization and their reaction equations
矿化原料 矿化的主要化学成分 化学方程式 石灰窑粉尘 CaO、MgO CaO+CO2→CaCO3
MgO+CO2→MgCO3煤粉灰 CaO CaO+CO2→CaCO3 钢渣 CaO、MgO CaO+CO2→CaCO3
MgO+CO2→MgCO3电石渣 Ca(OH)2、Mg(OH)2 Ca(OH)2+CO2→CaCO3+H2O
Mg(OH)2+CO2→MgCO3+H2O白泥 Ca(OH)2、Mg(OH)2、CaO Ca(OH)2+CO2→CaCO3+H2O
Mg(OH)2+CO2→MgCO3+H2O
CaO+CO2→CaCO3盐泥 Mg(OH)2 Mg(OH)2+CO2→MgCO3+H2O 电石废水 Ca(OH)2 Ca(OH)2+CO2→CaCO3+H2O 纺织、印染废水 NaOH NaOH+CO2→Na2CO3+H2O 盐湖苦卤 MgCl2 2NH3·H2O+CO2→(NH4)2CO3+H2O
MgCl2·6H2O+(NH4)2CO3→
MgCO3·3H2O+2NH4Cl+3H2O
5MgCl2·6H2O+5(NH4)2CO3→
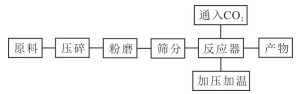
Mg(OH)2·4H2O+10NH4Cl+CO2+26H2O磷石膏 CaSO4·H2O CaSO4·H2O+2NH3·H2O+CO2→(NH4)2SO4+CaCO3+3H2O 表 2 不同矿化原料矿化反应方程式及最优条件
Table 2. Equations and optimum conditions for mineralization reaction of different mineralized raw materials
原料 媒介 方程式 最优条件 橄榄石 NaCl 2NaCl+2H2O→2NaOH+Cl2+H2
Cl2(g)+H2→2HCl; 4HCl+Mg2SiO4→2MgCl2+SiO2+2H2O
CO2+NaOH→NaHCO3; MgCl2+2NaHCO3→MgCO3+2NaCl+H2O+CO2粒径 < 110 μm
t=90 ℃
盐酸浓度 < 3 mol/L蛇纹石 NaCl 2NaCl+2H2O→2NaOH+H2+Cl2
Cl2+H2O→HCl+HClO
2HClO→2HCl+O2
6HCl+Mg3SiO5(OH)4→3MgCl2+2SiO2+5H2O
CO2+NaOH→NaHCO3
MgCl2+2NaHCO3→MgCO3+2NaCl+H2O+CO2p=4 MPa
t=150 ℃
粒径 < 30 μm硅灰石 HAc CaSiO3+2H+→Ca2++H2O+SiO2
CO2+H2O→H2CO3→H++HCO3-
Ca2++HCO3-→CaCO3+H+p=4 MPa
t=150 ℃
粒径 < 30 μm水镁石 NH4Cl Mg(OH)2+2NH4Cl→MgCl2+2NH3+2H2O
NH3+H2O→NH4OH
NH4OH+CO2→NH4HCO3
2NH4HCO3+MgCl2+2H2O→MgCO3·3H2O+2NH4Cl+CO2粒径 < 91 μm
t=100 ℃
氯化铵3 mol/L磷石膏 NH3·H2O CaSO4·H2O+2NH3·H2O+CO2→(NH4)2SO4+CaCO3+3H2O t=65 ℃、
固液比3.0、
氮硫比2.25氯化镁 NH3·H2O 2NH3·H2O+CO2→(NH4)2CO3+H2O
MgCl2·6H2O+(NH4)2CO3→MgCO3·3H2O+2NH4Cl+3H2O
5MgCl2·6H2O+5(NH4)2CO3→Mg(OH)2·4H2O+10NH4Cl+CO2+26H2Ot=40 ℃、
氯氨比1.0、乙醇体积分数30% -
Al, T. A., Martin, C. J., Blowes, D. W., 2000. Carbonate-Mineral/Water Interactions in Sulfide-Rich Mine Tailings. Geochimica et Cosmochimica Acta, 64(23):3933-3948. https://doi.org/10.1016/s0016-7037(0)00483-x Alexander, G., Mercedes Maroto-Valer, M., Gafarova-Aksoy, P., 2007. Evaluation of Reaction Variables in the Dissolution of Serpentine for Mineral Carbonation. Fuel, 86(1/2):273-281. https://doi.org/10.1016/j.fuel.2006.04.034 Bachu, S., Adams, J. J., 2003. Sequestration of CO2 in Geological Media in Response to Climate Change:Capacity of Deep Saline Aquifers to Sequester CO2 in Solution. Energy Conversion and Management, 44(20):3151-3175. https://doi.org/10.1016/s0196-8904(3)00101-8 Baciocchi, R., Polettini, A., Pomi, R., et al., 2006. CO2 Sequestration by Direct Gas-Solid Carbonation of Air Pollution Control (APC) Residues. Energy & Fuels, 20(5):1933-1940. https://doi.org/10.1021/ef060135b Berndt, M. E., Allen, D. E., Seyfried, W. E., 1996. Reduction of CO2 during Serpentinization of Olivine at 300℃ and 500 Bar. Geology, 24(4):351. https://doi.org/10.1130/0091-7613(1996)024 < 0351:rocdso > 2.3.co; 2 doi: 10.1130/0091-7613(1996)024<0351:rocdso>2.3.co;2 Black, B. A., Gibson, S. A., 2019. Deep Carbon and the Life Cycle of Large Igneous Provinces. Elements, 15(5):319-324. https://doi.org/10.2138/gselements.15.5.319 Blencoe, J. G., Palmer, D. A., Beard, J. S. 2004. Carbonation of Calcium Silicates for Long-Term CO2 Sequestration. WO, 2004094043, 2004-11-04. Brame, H. M. R., Martindale, R. C., Ettinger, N. P., et al., 2019. Stratigraphic Distribution and Paleoecological Significance of Early Jurassic (Pliensbachian-Toarcian) Lithiotid-Coral Reefal Deposits from the Central High Atlas of Morocco. Palaeogeography, Palaeoclimatology, Palaeoecology, 514:813-837. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9aef4736c5a4e629fbb3c57ffaf85cb3 Broecker, W. S., Takahashi, T., Simpson, H. J., et al., 1979. Fate of Fossil Fuel Carbon Dioxide and the Global Carbon Budget. Science, 206(4417):409-418. https://doi.org/10.1126/science.206.4417.409 Cui, X. Q., Bianchi, T. S., Savage, C., et al., 2016. Organic Carbon Burial in Fjords:Terrestrial Versus Marine Inputs. Earth and Planetary Science Letters, 451:41-50. Cui, Z. D., Liu, D. A., Zeng, R. S., et al., 2010. Geological Sequestration of CO2 and China's Sustainable Development. China Population Resources and Environment, 20(3):9-13 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgrkzyyhj201003002 Daval, D., Sissmann, O., Menguy, N., et al., 2011. Influence of Amorphous Silica Layer Formation on the Dissolution Rate of Olivine at 90℃ and Elevated PCO2. Chemical Geology, 284(1/2):193-209. https://doi.org/10.1016/j.chemgeo.2011.02.021 Du, Y. K., Pang, F., Chen, K., et al., 2019. Experiment of Breaking Shale Using Supercritical Carbon Dioxide Jet. Earth Science, 44(11):3749-3756 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dqkx201911014 Elderfield, H., 2010. Seawater Chemistry and Climate. Science, 327(5969):1092-1093. https://doi.org/10.1126/science.1186769 Etheridge, D. M., Steele, L. P., Langenfelds, R. L., et al., 1996. Natural and Anthropogenic Changes in Atmospheric CO2 over the Last 1 000 Years from Air in Antarctic Ice and Firn. Journal of Geophysical Research:Atmospheres, 101(D2):4115-4128. https://doi.org/10.1029/95jd03410 Fang, Q., Hong, H. L., Zhao, L. L., et al., 2018. Climatic Implication of Authigenic Minerals Formed during Pedogenic Weathering Processes. Earth Science, 43(3):753-769 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dqkx201803007 Fang, X. M., Galy, A., Yang, Y. B., et al., 2019. Paleogene Global Cooling-Induced Temperature Feedback on Chemical Weathering, as Recorded in the Northern Tibetan Plateau. Geology, 47(10):992-996. https://doi.org/10.1130/g46422.1 Gao, X., Meng, Y., Zhu, C., et al., 2011. Study on the Kinetics of Extracting Chrysotile with Ammonium Chloride. Carsologica Sinica, 30(4):472-478 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgyr201104020 Gao, X., Zhu, C., Zhao, L., 2012. Impact of Heat-Pretreatment on the Reactivity between Ammonium Chloride and Chrysotile. Geological Journal of China Universities, 18(2), 83-89 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gxdzxb201202009 Gerdemann, S. J., O'Connor, W. K., Dahlin, D. C., et al., 2007. Ex Situ Aqueous Mineral Carbonation. Environmental Science & Technology, 41(7):2587-2593. https://doi.org/10.1021/es0619253 Gislason, S. R., Oelkers, E. H., 2014. Carbon Storage in Basalt. Science, 344(6182):373-374. https://doi.org/10.1126/science.1250828 Goff, F., Lackner, K. S., 1998. Carbon Dioxide Sequestering Using Ultramaf Ic Rocks. Environmental Geosciences, 5(3):89-102. https://doi.org/10.1046/j.1526-0984.1998.08014.x Han, Z., Hu, X. M., Kemp, D. B., et al., 2018. Carbonate-Platform Response to the Toarcian Oceanic Anoxic Event in the Southern Hemisphere:Implications for Climatic Change and Biotic Platform Demise. Earth and Planetary Science Letters, 489:59-71. Hanchen, M., Prigiobbe, V., Baciocchi, R., et al., 2008. Precipitation in the Mg-Carbonate System-Effects of Temperature and CO2 Pressure. Chemical Engineering Science, 63(4):1012-1028. https://doi.org/10.1016/j.ces.2007.09.052 Hemmati, A., Shayegan, J., Sharratt, P., et al., 2014. Solid Products Characterization in a Multi-Step Mineralization Process. Chemical Engineering Journal, 252:210-219. https://doi.org/10.1016/j.cej.2014.04.112 Holloway, S., 1997. An Overview of the Underground Disposal of Carbon Dioxide. Energy Conversion and Management, 38:S193-S198. https://doi.org/10.1016/s0196-8904(96)00268-3 Houghton, J.T., Jenkins, G. J., Ephramus, J. J., 1992. Climate Change, the Supplementary Report to the IPCC Scientific Assessment. Cambridge University Press, Cambrige, 200. https://doi.org/10.1016/S0021-9169(96)90059-8 Houghton, R. A., Hackler, J. L., Lawrence, K. T., 1999. The U.S. Carbon Budget:Contributions from Land-Use Change. Science, 285(5427):574-78. https://doi.org/10.1126/science.285.5427.574 Hovelmann, J., Putnis, C. V., Ruiz-Agudo, E., et al., 2012. Direct Nanoscale Observations of CO2 Sequestration during Brucite[Mg(OH)2] Dissolution. Environmental Science & Technology, 46(9):5253-5260. https://doi.org/10.1021/es300403n Huijgen, W. J. J., Witkamp, G. J., Comans, R. N. J., 2006. Mechanisms of Aqueous Wollastonite Carbonation as a Possible CO2 Sequestration Process. Chemical Engineering Science, 61(13):4242-4251. https://doi.org/10.1016/j.ces.2006.01.048 Huntzinger, D. N., 2009. Carbon Dioxide Sequestration in Cement Kiln Dust through Miner Carbonation. Environmental Science & Technology, 43(6):1986-1992. https://doi.org/10.1021/es802910z Huntzinger, D. N., Gierke, J. S., Sutter, L. L., et al., 2009. Mineral Carbonation for Carbon Sequestration in Cement Kiln Dust from Waste Piles. Journal of Hazardous Materials, 168(1):31-37. https://doi.org/10.1016/j.jhazmat.2009.01.122 Izumi, K., Kemp, D. B., Itamiya, S., et al., 2018. Sedimentary Evidence for Enhanced Hydrological Cycling in Response to Rapid Carbon Release during the Early Toarcian Oceanic Anoxic Event. Earth and Planetary Science Letters, 481:162-170. https://doi.org/10.1016/j.epsl.2017.10.030 Joos, F., 1994. Imbalance in the Budget. Nature, 370(6486):181-182. https://doi.org/10.1038/370181a0 Kakizawa, M., Yamasaki, A., Yanagisawa, Y., 2001. A New CO2 Disposal Process Via Artificial Weathering of Calcium Silicate Accelerated by Acetic Acid. Energy, 26(4):341-354. https://doi.org/10.1016/s0360-5442(1)00005-6 Kasting, J., 1984. Comments on the BLAG Model; The Carbonate-Silicate Geochemical Cycle and Its Effect on Atmospheric Carbon Dioxide over the Past 100 Million Years. American Journal of Science, 284(10):1175-1182. https://doi.org/10.2475/ajs.284.10.1175 Katsuyama, Y., Yamasaki, A., 2010. Development of a Process for Producing High-Purity Calcium Carbonate (CaCO3) from Waste Cement Using Pressurized CO2. Environmental Progress, 24(2):162-170. https://doi.org/10.1002/ep.10080 Kelemen, P. B., Matter, J., 2008. In Situ Carbonation of Peridotite for CO2 Storage. Proceedings of the National Academy of Sciences, 105(45):17295-17300. https://doi.org/10.1073/pnas.0805794105 King, H. E., Satoh, H., Tsukamoto, K., et al., 2014. Surface-Specific Measurements of Olivine Dissolution by Phase-Shift Interferometry. American Mineralogist, 99(2/3):377-386. https://doi.org/10.2138/am.2014.4606 Lackner, K. S., Butt, D. P., Wendt, C. H., 1997. Progress on Binding CO2 in Mineral Substrates. Energy Conversion and Management, 38:S259-S264. https://doi.org/10.1016/s0196-8904(96)00279-8 Lackner, K. S., Wendt, C. H., Butt, D. P., et al., 1995. Carbon Dioxide Disposal in Carbonate Minerals. Energy, 20(11):1153-1170. https://doi.org/10.1016/0360-5442(95)00071-n Lekakh, S. N., Robertson, D. G. C., Rawlins, C. H., et al., 2008. Investigation of a Two-Stage Aqueous Reactor Design for Carbon Dioxide Sequestration Using Steelmaking Slag. Metallurgical and Materials Transactions B, 39(3):484-492. https://doi.org/10.1007/s11663-008-9155-5 Li, C. J., Wang, S. J., Bai, X. Y., et al., 2019. Estimation of Carbonate Rock Weathering-Related Carbon Sink in Global Major River Basins. Acta Geographica Sinica, 74(7):1319-1332 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dlxb201907005 Li, H. W., Wang, S. J., Bai, X. Y., et al., 2018. Spatiotemporal Distribution and National Measurement of the Global Carbonate Carbon Sink. Science of the Total Environment, 643:157-170. https://doi.org/10.1016/j.scitotenv.2018.06.196 Li, H. W., Wang, S. J., Bai, X. Y., et al., 2019. Spatiotemporal Evolution of Carbon Sequestration of Limestone Weathering in China. Science China Earth Sciences, 62(6):974-991. https://doi.org/10.1007/s11430-018-9324-2 Li, H. W., Wang, S. J., Bai, Y. X., et al., 2019. Effects of Climate Change and Ecological Restoration on Carbonate Rock Weathering Carbon Sequestration in the Karst Valley of Southwest China. Acta Ecologica Sinica, 39(16):6158-6172 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=stxb201916037 Li, Q., Cai, B. F., Chen, F., et al., 2019. Review of Environmental Risk Assessment Methods for Carbon Dioxide Geological Storage. Environmental Engineering, 37(2):16-24 (in Chinese with English abstract). Li, W. Z., Li, W., Bai, Z. Q., et al., 2010. Sequestration of Carbon Dioxide with Olivine Promoted by an Electrochemical Method. Journal of China University of Mining & Technology, 39(2):265-269 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgkydxxb201002022 Li, W. Z., Li, W., Li, B. Q., et al., 2007. Using Electrolytic Method to Promote CO2 Sequestration in Serpentine by Mineral Carbonation. Journal of China University of Mining & Technology, 36(6):817-821 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgkydxxb200706020 Li, Y., Bai, X. Y., Wang, S. J., et al., 2017. Evaluating of the Spatial Heterogeneity of Soil Loss Tolerance and its Effects on Erosion Risk in the Carbonate Areas of Southern China. Solid Earth, 8(3):661-669. https://doi.org/10.5194/se-8-661-2017 Li, Z. B., Liu, L. W., Zhao, L., et al., 2011. Carbon Dioxide Sequestration by Ultramafic-Hosted Mine Tailings:Example from Jinchuan Copper-Nickel Mine Tailing. Quaternary Sciences, 31(3):70-78 (in Chinese with English abstract). Liu, X. Y., Ding, C. X., Chu, P. K., 2004. Mechanism of Apatite Formation on Wollastonite Coatings in Simulated Body Fluids. Biomaterials, 25(10):1755-1761. https://doi.org/10.1016/j.biomaterials.2003.08.024 Liu, Z. H., 2012. New Progress and Prospects in the Study of Rock-Weathering-Related Carbon Sinks. Chinese Science Bulletin, 57(2), 95-102 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kxtb201202001 Liu, Z. H., Dreybrodt, W., Liu, H., 2011. Atmospheric CO2 Sink:Silicate Weathering or Carbonate Weathering?. Quaternary Sciences, 31(3):32-36 (in Chinese with English abstract). Liu, Z. M., Wu, Y. H., 2015. Geological and Current Development Utilization of Serpentinite, China. Geology of Chemical Minerals, 37(3):171-179 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgkcdz201503011 Liu, Z. Q., Hao, Z. G., Liu, L., et al., 2016. Status of the Comprehensive Utilization of Tailings in China and Suggestions. Geological Review, 62(5):1277-1282 (in Chinese). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgky201405005 Lizuka, A., Fujii, M., Yamasaki, A., et al., 2004. Development of a New CO2, Sequestration Process Utilizing the Carbonation of Waste cement. Industrial & Engineering Chemistry Research, 43(24):7880-7887. https://doi.org/10.1021/ie0496176 Lokhorst, A., Wildenborg, T., 2005. Introduction on CO2 Geological Storage-Classification of Storage Options. Oil & Gas Science and Technology, 60(3):513-515. https://doi.org/10.2516/ogst:2005033 Macdonald, F. A., Swanson-Hysell, N. L., Park, Y., et al., 2009. Arc-Continent Collisions in the Tropics Set Earth's Climate State. Science, 364(6436):181-84. https://doi.org/10.1126/science.aav5300 Marchetti, C., 1977. On Geoengineering and the CO2 Problem. Climatic Change, 1(1):59-68. https://doi.org/10.1007/bf00162777 Maroto-Valer, M. M., Fauth, D. J., Kuchta, M. E., et al., 2005. Activation of Magnesium Rich Minerals as Carbonation Feedstock Materials for CO2 Sequestration. Fuel Processing Technology, 86(14/15):1627-1645. https://doi.org/10.1016/j.fuproc.2005.01.017 Mckelvy, M. J., Bearat, H., Chizmeshya, A. V. G., et al., 2003. Understanding Olivine CO2 Mineral Sequestration Mechanisms at the Atomic Level:Optimizing Reaction Process Design. Office of Scientific & Technical Information Technical Reports, 20(1-3):514-524. https://doi.org/10.2172/822896 Metz, B., Davidson, O., Connick, H. D., et al., 2005. Report on Carbon Dioxide Capture and Storage. Cambridge University Press, Cambridge. Meyer, N. A., Vögeli, J. U., Becker, M., et al., 2014. Mineral Carbonation of PGM Mine Tailings for CO2 Storage in South Africa:A Case Study. Minerals Engineering, 59:45-51. https://doi.org/10.1016/j.mineng.2013.10.014 Miilar, C. M., Aduomih, A. A. O., Still, B., et al., 2015. Estuarine Subaqueous Soil Organic Carbon Accounting:Sequestration and Storage. Soil Science Society of America Journal, 79 (2):389-397. https://doi.org/10.2136/sssaj2014.05.0204 Monger, H. C., Kraimer, R. A., Khresat, S., et al., 2015. Sequestration of Inorganic Carbon in Soil and Groundwater. Geology, 43(5):375-378. https://doi.org/10.1130/g36449.1 Montes-Hernandez, G., Pérez-López, R., Renard, F., et al., 2009. Mineral Sequestration of CO2 by Aqueous Carbonation of Coal Combustion Fly-Ash. Journal of Hazardous Materials, 161(2/3):1347-1354. https://doi.org/10.1016/j.jhazmat.2008.04.104 Neftel, A., Oeschger, H., Schwander, J., et al., 1982. Ice Core Sample Measurements Give Atmospheric CO2 Content during the Past 40 000 Yr. Nature, 295(5846):220-223. https://doi.org/10.1038/295220a0 O'Connor, W. K., Dahlin, D. C., Rush, G. E., et al., 2000. Carbon Dioxide Sequestration by Direct Mineral Carbonation:Process Mineralogy of Feed and Products. Mining, Metallurgy & Exploration, 19(2):95-101. https://doi.org/10.1007/bf03403262 O'Connor, W. K., Dahlin, D. C., Rush, G. E., et al., 2004. Energy and Economic Considerations for Ex-Situ and Aqueous Mineral Carbonation. Coal Technology Association Suffield Drive Gaithersburg Md, New York. Olajire, A. A., 2013. A Review of Mineral Carbonation Technology in Sequestration of CO2. Journal of Petroleum Science and Engineering, 109:364-392. https://doi.org/10.1016/j.petrol.2013.03.013 Olsson, J., Bovet, N., Makovicky, E., et al., 2012. Olivine Reactivity with CO2 and H2O on a Microscale:Implications for Carbon Sequestration. Geochimica et Cosmochimica Acta, 77:86-97. https://doi.org/10.1016/j.gca.2011.11.001 Paktunc, A. D., Davé, N. K., 2002. Formation of Secondary Pyrite and Carbonate Minerals in the Lower Williams Lake Tailings Basin, Elliot Lake, Ontario, Canada. American Mineralogist, 87(5/6):593-602. https://doi.org/10.2138/am-2002-5-601 Pan, X., 2007. Experimental and Carbonation Mechanism Study on Silicate for CO2 Sequestration(Dissertation). Huazhong University of Science & Technology, Wuhan, 32 (in Chinese with English abstract). Petit, J. R., Jouzel, J., Raynaud, D., et al., 1999. Climate and Atmospheric History of the Past 420, 000 Years from the Vostok Ice Core, Antarctica. Nature, 399(6735):429-436. https://doi.org/10.1038/20859 Peuble, S., Andreani, M., Godard, M., et al., 2015. Carbonate Mineralization in Percolated Olivine Aggregates:Linking Effects of Crystallographic Orientation and Fluid Flow. American Mineralogist, 100(2/3):474-482. https://doi.org/10.2138/am-2015-4913 Rendek, E., Ducom, G., Germain, P., 2006. Carbon Dioxide Sequestration in Municipal Solid Waste Incinerator (MSWI) Bottom Ash. Journal of Hazardous Materials, 128(1):73-79. https://doi.org/10.1016/j.jhazmat.2005.07.033 Rollo, H. A., Jamieson, H. E., 2006. Interaction of Diamond Mine Waste and Surface Water in the Canadian Arctic. Applied Geochemistry, 21(9):1522-1538. https://doi.org/10.1016/j.apgeochem.2006.05.008 Romanov, V., Soong, Y., Carney, C., et al., 2015. Mineralization of Carbon Dioxide:A Literature Review. Chem. Bio. Eng. Reviews, 2(4):231-256. https://doi.org/10.1002/cben.201500002 Sanna, A., Uibu, M., Caramanna, G., et al., 2014. A Review of Mineral Carbonation Technologies to Sequester CO2. Chem. Soc. Rev., 43(23):8049-8080. https://doi.org/10.1039/c4cs00035h Schaef, H. T., Windisch, C. F. Jr, McGrail, B. P., et al., 2011. Brucite[Mg(OH)2] Carbonation in Wet Supercritical CO2:An in Situ High Pressure X-Ray Diffraction Study. Geochimica et Cosmochimica Acta, 75(23):7458-7471. https://doi.org/10.1016/j.gca.2011.09.029 Schuiling, R. D., Krijgsman, P., 2006. Enhanced Weathering:An Effective and Cheap Tool to Sequester CO2. Climatic Change, 74(1/2/3):349-354. https://doi.org/10.1007/s10584-005-3485-y Schwartzman, D. W., Volk, T., 1989. Biotic Enhancement of Weathering and the Habitability of Earth. Nature, 340(6233):457-460. https://doi.org/10.1038/340457a0 Seifritz, W., 1990. CO2 Disposal by Means of Silicates. Nature, 345(6275):486-486. https://doi.org/10.1038/345486b0 Sheng, X. F., Ji, J. F., Chen, J., 2011. Assessment of Carbon Dioxide Sequestration Potential of Ultramafic Rocks in China. Quaternary Sciences, 31(3):447-454 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dsjyj201103007 Smith, R. W., Bianchi, T. S., Allison, M., et al., 2015. High Rates of Organic Carbon Burial in Fjord Sediments Globally. Nature Geoscience, 8(6):450-453. https://doi.org/10.1038/ngeo2421 Steel, K. M., Alizadehhesari, K., Balucan, R. D., et al., 2013. Conversion of CO2 into Mineral Carbonates Using a Regenerable Buffer to Control Solution pH. Fuel, 111:40-47. https://doi.org/10.1016/j.fuel.2013.04.033 Stolaroff, J. K., Lowry, G. V., Keith, D. W., 2005. Using CaO- And MgO-Rich Industrial Waste Streams for Carbon Sequestration. Energy Conversion and Management, 46(5):687-699. https://doi.org/10.1016/j.enconman.2004.05.009 Suarez, C. A., Edmonds, M., Jones, A. P., 2019. Earth Catastrophes and their Impact on the Carbon Cycle. Elements, 15(5):301-306. https://doi.org/10.2138/gselements.15.5.301 Tanaka, K., Okawa, H., Hashimoto, K., et al., 2016. Effect of NO2 in Exhaust Gas from an Oxyfuel Combustion System on the Cap Rock of a Proposed CO2 Injection Site. Applied Geochemistry, 70:17-26. https://doi.org/10.1016/j.apgeochem.2016.04.007 Tang, H. Y., Meng, W. J., Sun, S. H., et al., 2014. Leaching and Carbonation of Steelmaking Slag. Journal of University of Science and Technology Beijing, 8(S1):27-31 (in Chinese with English abstract). Tang, L., 2017. Natural CO2 Mineralization with V-Ti-Fe Ore Tailings in Panxi Region(Dissertation). Sichuan University, Chengdu, 23 (in Chinese with English abstract). Teir, S., Eloneva, S., Fogelholm, C. J., et al., 2007. Dissolution of Steelmaking Slags in Acetic Acid for Precipitated Calcium Carbonate Production. Energy, 32(4):528-539. https://doi.org/10.1016/j.energy.2006.06.023 Uibu, M., Uus, M., Kuusik, R., 2009. CO2 Mineral Sequestration in Oil-Shale Wastes from Estonian Power Production. Journal of Environmental Management, 90(2):1253-1260. https://doi.org/10.1016/j.jenvman.2008.07.012 Vogeli, J., Reid, D. L., Becker, M., et al., 2011. Investigation of the Potential for Mineral Carbonation of PGM Tailings in South Africa. Minerals Engineering, 24(12):1348-1356. https://doi.org/10.1016/j.mineng.2011.07.005 Wang, S. J., Liu, Z. H., Ni, J., et al., 2017. A Review of Research Progress and Future Prospective of Carbon Cycle in Karst Area of South China. Earth and Environment, 45(1):2-9 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dzdqhx201701002 Wang, X. L., Maroto-Valer, M. M., 2013. Optimization of Carbon Dioxide Capture and Storage with Mineralisation Using Recyclable Ammonium Salts. Energy, 51:431-438. https://doi.org/10.1016/j.energy.2013.01.021 Wang, Y. F., 2015. An Exploratory Study on CO2 Mineralization and Utilization(Dissertation). Sichuan University, Chengdu, 14 (in Chinese with English abstract). Wang, Z. H., Zhang, J. Y., Xu, J., et al., 2008. A Theoretical Study on Mineral Carbonation for CO2 Sequestration. Journal of Engineering Thermophysics, 29(6):1063-1068 (in Chinese with English abstract). Wendt, C. H., Butt, D. P., Lackner, K. S., et al., 1999. Thermodynamic Considerations of Using Chlorides to Accelerate the Carbonate Formation from Magnesium Silicates. Los Alamos Nation Laboratory, Los Alamos, 349-354. Weng, J. T., 1995. The Effect of Carbonate Rocks on Global Carbon Cycle. Advance in Earth Sciences, 10(2):154-158 (in Chinese with English abstract). Wilson, S. A., 2006. Verifying and Quantifying Carbon Fixation in Minerals from Serpentine-Rich Mine Tailings Using the Rietveld Method with X-Ray Powder Diffraction Data. American Mineralogist, 91(8/9):1331-1341. https://doi.org/10.2138/am.2006.2058 Wilson, S. A., Dipple, G. M., 2009. Quantifying Carbon Fixation in Trace Minerals from Processed Kimberlite:A Comparative Study of Quantitative Methods Using X-Ray Powder Diffraction Data with Applications to the Diavik Diamond Mine, Northwest Territories, Canada. Applied Geochemistry, 24(12):2312-2331. https://doi.org/10.1016/j.apgeochem.2009.09.018 Wilson, S. A., Dipple, G. M., Power, I. M., et al., 2009. Carbon Dioxide Fixation within Mine Wastes of Ultramafic-Hosted Ore Deposits:Examples from the Clinton Creek and Cassiar Chrysotile Deposits, Canada. Economic Geology, 104(1):95-112. https://doi.org/10.2113/gsecongeo.104.1.95 Wu, H. Z., 2011. Summary of Study of Solid Waste Carbonation. Coal Ash China, 23(1):33-35 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fmh201101010 Xie, H. P., 2010. Developing Low-Carbon Technology and Promoting Green Economy. Energy of China, 32(9):5-10 (in Chinese with English abstract). Xie, H. P., Wang, Y. F., Chu, W., et al., 2014. Mineralization of Flue Gas CO2 with Coproduction of Valuable Magnesium Carbonate by Means of Magnesium Chloride. Chinese Science Bulletin, 59(23):2882-2889. https://doi.org/10.1007/s11434-014-0388-1 Xie, H. P., Xie, L. Z., Wang, Y. F., et al., 2012. CCU:A More Feasible and Economic Strategy than CCS for Reducing CO2 Emissions. Journal of Sichuan University (Engineering Science Edition), 44(4):1-5 (in Chinese with English abstract). Xu, J., 2006. Experimental Study on the Reaction Mechanism of Carbon dioxide Mineral Carbonation. Huazhong University of Science and Technology, 19 (in Chinese with English abstract). Yadav, V. S., Prasad, M., Khan, J., et al., 2010. Sequestration of Carbon Dioxide (CO2) Using Red Mud. Journal of Hazardous Materials, 176(1/2/3):1044-1050. https://doi.org/10.1016/j.jhazmat.2009.11.146 Yan, H., Zhang, J. Y., Wang, Z. L., et al., 2013. CO2 Sequestration by Direct Mineral Carbonation of Serpentine under Medium and Low Pressure. Journal of Fuel Chemistry and Technology, 41(6):748-753 (in Chinese with English abstract). Yao, R., 2003. Research of Carbon Sink Capacity Caused by Rock Weathering Process in China. Central South University, Changsha, 7 (in Chinese with English abstract). Yu, G., Song, C., Pan, Y., et al., 2014. Review of New Progress in Tailing Dam Safety in Foreign Research and Current State with Development Trent in China (in Chinese). Chinese Journal of Rock Mechanics and Engineering, 33(2014):3238-3248. Yuan, D. X., 2001. Carbon Cycle in Earth System and Its Effects on Environment and Resources. Quaternary Sciences, 21(3):223-232 (in Chinese with English abstract). Zeng, Q. R., Liu, Z. H., 2017. Is Basalt Weathering a Major Mechanism for Atmospheric CO2 Consumption? Chinese Science Bulletin, 62(10):1041-1049(in Chinese with English abstract). Zhang, B. B., Wang, H. M., Zeng, S. H., et al., 2012. Current Status and Outlook of Carbon Dioxide Mineral Carbonation Technologies. Chemical Industry and Engineering Progress, 31(9):2075-2083 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201209044 Zhang, J. S., Zhang, R., Bi, J. C., 2011. Fundamental Research on CO2 Mineralization:I.Leaching Kinetics of Forsterite and Serpentine with Hydrochloric Acid. Journal of Fuel Chemistry and Technology, 39(9):706-711 (in Chinese with English abstract). Zhang, J., Zhang, R., Geerlings, H., et al., 2012. Mg-Silicate Carbonation Based on an HCl- and NH3- Recyclable Process:Effect of Carbonation Temperature. Chemical Engineering & Technology, 35(3):525-531. https://doi.org/10.1002/ceat.201100425 Zhao, L., Sang, L. Q., Chen, J., et al., 2010. Aqueous Carbonation of Natural Brucite:Relevance to CO2 Sequestration. Environmental Science & Technology, 44(1):406-411. https://doi.org/10.1021/es9017656 Zhao, Y. M., Feng, C. Y., Li, D. X., 2017. New Progress in Prospecting for Skarn Deposits and Spatial-Teporal Distribution of Skarn Deposits in China. Mineral Deposits, 36(3):519-543 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kcdz201703001 Zhou, J. P., Xian, X. F., Jiang, Y. D., et al., 2010. A Permeability Model Including Effective Stress and Coal Matrix Shrinking Effect. Rock and Soil Mechanics, 31(7):2317-2323 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ytlx201007049 Zhu, C., Zhao, L., Gao, X., et al., 2011. CO2 Sequestration Based Study of Reaction Kinetics of Brucite. Quaternary Sciences, 31(3):438-446 (in Chinese with English abstract). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dsjyj201103006 Zimmerman, A. R., Cornelissen, G., 2018. Consider Fjord-Assisted Carbon Storage. Environmental Science & Technology, 52(19):10911-10913. https://doi.org/10.1021/acs.est.8b04854 Zoback, M. D., Gorelick, S. M., 2012. Earthquake Triggering and Large-Scale Geologic Storage of Carbon Dioxide. Proceedings of the National Academy of Sciences, 109(26):10164-10168. https://doi.org/10.1073/pnas.1202473109 曾庆睿, 刘再华, 2017.玄武岩风化是重要的碳汇机制吗?.科学通报, 62(10):1041-1049. 崔振东, 刘大安, 曾荣树, 等, 2010.中国CO2地质封存与可持续发展.中国人口·资源与环境, 20(3):9-13. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgrkzyyhj201003002 杜玉昆, 庞飞, 陈科, 等, 2019.超临界二氧化碳喷射破碎页岩试验.地球科学, 44(11):3749-3756. doi: 10.3799/dqkx.2019.221 方谦, 洪汉烈, 赵璐璐, 等, 2018.风化成土过程中自生矿物的气候指示意义.地球科学, 43(3):753-769. doi: 10.3799/dqkx.2018.905 高雄, 孟烨, 朱辰, 等, 2011.氯化铵浸取纤蛇纹石动力学研究.中国岩溶, 30(4):472-478. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgyr201104020 高雄, 朱辰, 赵良, 2012.灼烧处理对纤蛇纹石反应活性的影响.高校地质学报, 18(2):83-89. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gxdzxb201202009 李朝君, 王世杰, 白晓永, 等, 2019.全球主要河流流域碳酸盐岩风化碳汇评估.地理学报, 74(7):1319-1332. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dlxb201907005 李汇文, 王世杰, 白晓永, 等, 2019.气候变化及生态恢复对喀斯特槽谷碳酸盐岩风化碳汇的影响评估.生态学报, 39(16):6158-6172. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=stxb201916037 李琦, 蔡博峰, 陈帆, 等, 2019.二氧化碳地质封存的环境风险评价方法研究综述.环境工程, 37(2):16-24. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjgc201902003 李文志, 李文, 白宗庆, 等, 2010.电解法促进橄榄石固定CO2的研究.中国矿业大学学报, 39(2):265-269. 李文志, 李文, 李保庆, 等, 2007.电解法用于促进蛇纹石矿物固定CO2的研究.中国矿业大学学报, 36(6):817-821. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgkydxxb200706020 李子波, 刘连文, 赵良, 等, 2011.应用超基性岩尾矿封存CO2——以金川铜镍矿尾矿为例.第四纪研究, 31(3), 70-78. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dsjyj201103009 刘再华, 2012.岩石风化碳汇研究的最新进展和展望.科学通报, 57(2):95-102. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kxtb201202001 刘再华, Dreybrodt, W., 刘洹. 2011.大气CO2汇:硅酸盐风化还是碳酸盐风化的贡献?.第四纪研究, 31(3):32-36. 刘振敏, 吴颖慧, 2015.中国蛇纹岩矿地质特征及开发利用现状.化工矿产地质, 37(3): 171-179. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgkcdz201503011 刘志强, 郝梓国, 刘恋, 等, 2016.我国尾矿综合利用研究现状及建议.地质论评, 62(5):1277-1282. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=slsy201725171 倪健, 王世杰, 刘再华, 等, 2017.中国喀斯特碳循环.地球与环境, 45(1):1. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dzdqhx201701001 潘霞, 2007.硅酸盐碳酸化隔离CO2的实验和理论研究(博士学位论文).武汉: 华中科技大学, 32. 盛雪芬, 季峻峰, 陈骏, 2011.中国超基性岩封存CO2的潜力研究.第四纪研究, 31(3):447-454. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dsjyj201103007 唐海燕, 孟文佳, 孙绍恒, 等, 2014.炼钢炉渣的浸出和碳酸化.北京科技大学学报, 8(S1):27-31. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QKV20142015100800404482 唐亮, 2017.攀西地区尾矿自矿化利用CO2研究(硕士学位论文).成都: 四川大学, 23. 王世杰, 刘再华, 倪健, 等, 2017.中国南方喀斯特地区碳循环研究进展.地球与环境, 45(1):2-9. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dzdqhx201701002 王昱飞, 2015. CO2矿化利用探索研究(博士学位论文).成都: 四川大学, 15. 王宗华, 张军营, 徐俊, 等, 2008. CO2矿物碳酸化隔离的理论研究.工程热物理学报, 29(6):1063-1068. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gcrwlxb200806043 翁金桃, 1995.碳酸盐岩在全球碳循环过程中的作用.地球科学进展, 10(2):154-158. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199500054144 吴昊泽, 2011.固体废弃物碳酸化研究综述.粉煤灰, 23(1):33-35. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fmh201101010 谢和平, 2010.发展低碳技术推进绿色经济.中国能源, 32(9):5-10. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgny201009002 谢和平, 王昱飞, 储伟, 等, 2014.氯化镁矿化利用低浓度烟气CO2联产碳酸镁.科学通报, 59(19):1797-1803. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kxtb201419001 谢和平, 谢凌志, 王昱飞, 等, 2012.全球CO2减排不应是CCS, 应是CCU.四川大学学报(工程科学版), 44(4):1-5. 徐俊, 2006. CO2矿化机制的实验研究.华中科技大学, 19. 晏恒, 张军营, 王志亮, 等, 2013.中低压条件下蛇纹石直接矿物碳酸化隔离CO2的实验研究.燃料化学学报, 41(6):748-753. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201306017 姚锐, 2003.中国岩石风化对大气CO2的汇效应研究(硕士学位论文).长沙: 中南大学, 7. 袁道先, 2001.地球系统的碳循环和资源环境效应.第四纪研究, 21(3):223-232. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dsjyj200103004 张兵兵, 王慧敏, 曾尚红, 等, 2012. CO2矿物封存技术现状及展望.化工进展, 31(9):2075-2083. 张建树, 张荣, 毕继诚, 2011. CO2矿化反应基础研究Ⅰ:镁橄榄石和蛇纹石盐酸浸出动力学研究.燃料化学学报, 39(9):706-711. 张军营, 赵永椿, 潘霞, 等, 2008.硅灰石碳酸化隔离CO2的实验研究.自然科学进展, 18(7):836-840. 赵一鸣, 丰成友, 李大新, 2017.中国矽卡岩矿床找矿新进展和时空分布规律.矿床地质, 36(3):519-543. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kcdz201703001 周军平, 鲜学福, 姜永东, 等, 2010.考虑基质收缩效应的煤层气应力场-渗流场耦合作用分析.岩土力学, 31(7):2317-2323. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ytlx201007049 朱辰, 赵良, 高雄, 等, 2011.基于CO2封存的水镁石反应动力学研究.第四纪研究, 31(3):438-446. -









 下载:
下载: