Adsorption Characteristics of Cd in Alluvial and Lacustrine Soils: A Case Study in Dangtu County, Anhui Province
-
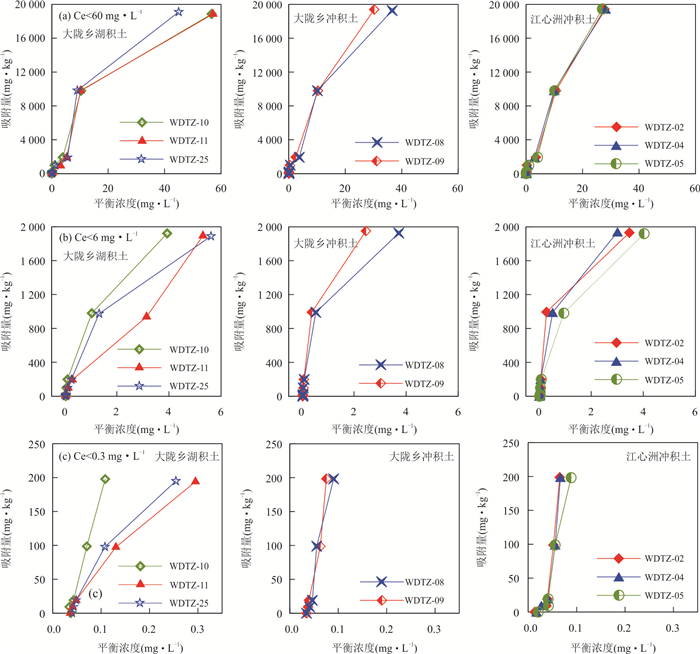
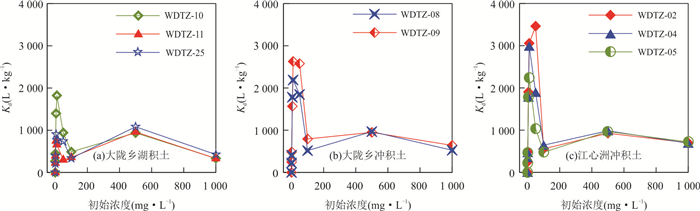
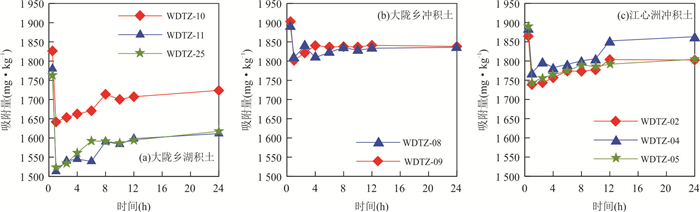
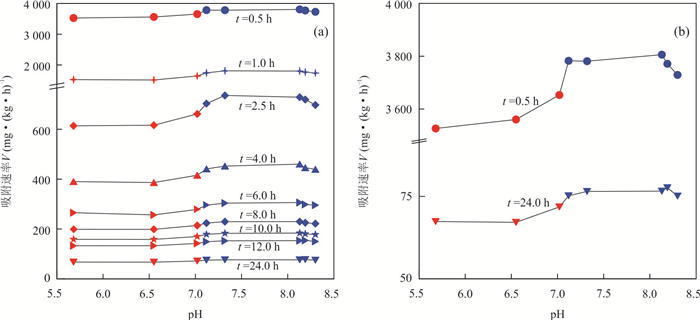
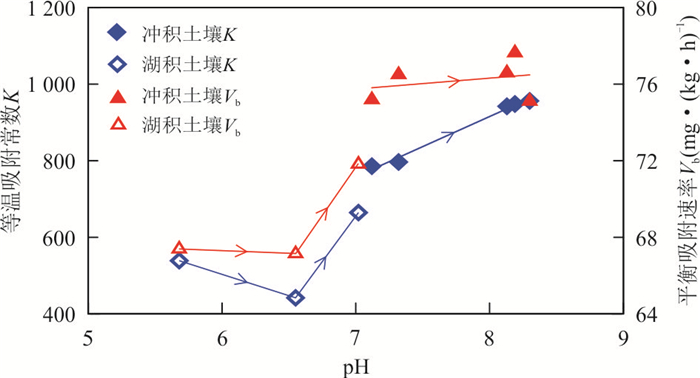
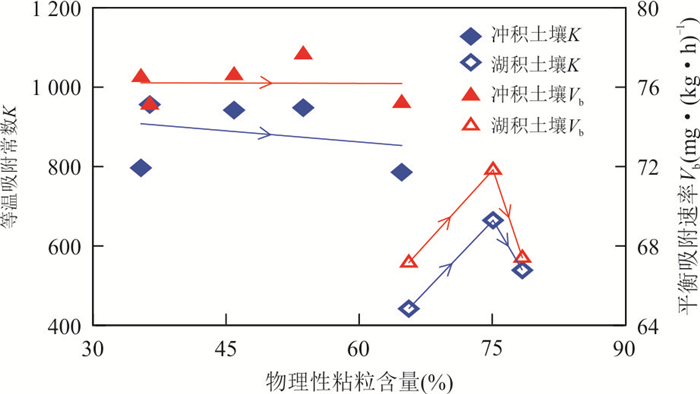
摘要: 为揭示冲积与湖积成因土壤镉的吸附特征,以安徽省当涂县冲积成因的江心洲和冲积、湖积成因的大陇乡根际土壤为研究对象,开展土壤镉的等温吸附实验和吸附动力学实验.等温吸附实验结果表明,冲积土壤镉的吸附量(S)、等温吸附常数(K)和固液分配系数(Kd)均较湖积土壤明显偏大,显示冲积土壤对镉的吸附能力较湖积土壤强;吸附动力学实验表明,冲积土壤的最大吸附量、平衡吸附量均较湖积土壤大,吸附速率也明显偏大,尤其在吸附实验早期更为显著;等温吸附常数K和平衡吸附速率Vb与土壤理化性质的分析表明,土壤pH是造成研究区土壤镉吸附能力差异的主要原因,其次为物理性粘粒含量;土壤pH是影响研究区冲积土壤镉吸附能力的主要因素;湖积土壤镉的吸附能力受土壤pH、有机质含量、Cd含量、物理性粘粒含量等因素的综合影响.研究对于揭示Cd在水土系统的迁移转化规律以及土壤Cd的污染防治具有重要的指导意义.Abstract: In order to reveal the adsorption characteristics of alluvial and lacustrine soil cadmium,in this paper it takes alluvial soils of Jiangxin Town and alluvial,lacustrine soils of Dalong Town as the research object in Dangtu County,Anhui Province. The isothermal adsorption experiment and adsorption kinetics experiment of soil cadmium were carried out. The results of isothermal adsorption experiments show that the soil cadmium adsorbance (S),the isothermal adsorption constant (K) and solid-liquid partition coefficient (Kd) of alluvial soils are significantly greater than those of the lacustrine soils,which indicates that the cadmium adsorption capacity of alluvial soils is stronger than that of lacustrine soils. The adsorption kinetics experiment shows that the largest adsorbance,balance adsorbance of soil cadmium in alluvial soils are bigger than those of the lacustrine soils,and the adsorption rate of alluvial soils is also higher than that of lacustrine soils,especially in the early stage of adsorption experiments. The correlation analysis between isothermal adsorption constant K,balance adsorption rate Vb and soil physical and chemical properties shows that soil pH is the main factor for the adsorption capacity of soil cadmium in the study area,which is followed by the physical clay content. Soil pH is the main factor of cadmium adsorption capacity for alluvial soils in the study area. The cadmium adsorption capacity for lacustrine soils is affected by pH,organic matter content,Cd content and the physical clay content synthetically in the study area. It has great significance to reveal the law of Cd migration and transformation in soil and water system and the prevention and control of Cd pollution in soil.
-
Key words:
- alluvial soil /
- lacustrine soil /
- Cd /
- adsorption characteristics /
- geochemistry
-
表 1 供试样品基本情况
Table 1. Basic information of test samples
沉积物类型 野外编号 pH 有机质(%) Cd (mg·kg-1) 粘粒(%) 粉粒(%) 砂粒(%) 作物类型 土壤质地 (< 2 μm) (2~20 μm) (> 20 μm) 大陇乡湖积物 WDTZ-10 7.02 1.52 0.39 10.04 78.04 11.93 水稻 粉砂质壤土 WDTZ-11 6.55 1.20 0.64 3.47 82.99 13.54 水稻 粉砂质壤土 WDTZ-25 5.68 1.44 0.30 16.34 72.99 10.68 水稻 粉砂质粘壤土 平均值 / 6.42 1.39 0.44 9.95 78.01 12.05 水稻 / 大陇乡冲积物 WDTZ-08 7.32 1.47 0.31 1.99 55.00 43.01 水稻 粉砂质壤土 WDTZ-09 8.13 0.95 0.28 1.11 70.34 28.55 水稻 粉砂质壤土 平均值 / 7.73 1.21 0.30 1.55 62.67 35.78 水稻 / 江心洲冲积物 WDTZ-02 8.30 0.76 0.28 1.83 58.07 40.10 玉米 粉砂质壤土 WDTZ-04 8.19 0.90 0.34 3.05 73.31 23.64 玉米 粉砂质壤土 WDTZ-05 7.12 1.52 0.46 6.44 76.40 17.16 玉米 粉砂质壤土 平均值 / 7.87 1.06 0.36 3.77 69.26 26.97 玉米 / 表 2 冲积和湖积土壤镉的等温吸附实验Freundlich方程拟合结果
Table 2. The fitting results of the isothermal adsorption by Freundlich model in alluvial and lacustrine soils
沉积物类型 野外编号 K 1/n R2 大陇乡湖积物 WDTZ-10 664.66 0.96 0.939 7** WDTZ-11 442.08 1.02 0.967 1** WDTZ-25 539.01 1.04 0.960 9** 平均值 548.58 1.00 0.955 9 大陇乡冲积物 WDTZ-08 796.71 0.99 0.911 6** WDTZ-09 942.11 1.00 0.913 0** 平均值 869.41 0.99 0.912 3 江心洲冲积物 WDTZ-02 956.31 0.97 0.887 9** WDTZ-04 948.20 0.98 0.927 7** WDTZ-05 785.78 1.01 0.926 1** 平均值 896.76 0.98 0.913 9 注:**在0.01水平(双侧)上显著相关;*在0.05水平(双侧)上显著相关,下表同. 表 3 冲积与湖积土壤镉的吸附动力学方程
Table 3. Adsorption kinetics equation of Cd2+ on alluvial and lacustrine soils
沉积物类型 模型 Elovich模型:S(t)=(1/β)lnαβ+(1/β)lnt 参数 α(mg·(kg·h)-1) β(kg·mg-1) R2 江心洲冲积物 WDTZ-02 1.37×1034 0.043 6 0.878 2** WDTZ-04 2.87×1024 0.030 3 0.760 0** WDTZ-05 3.37×1038 0.049 2 0.966 7** 大陇乡冲积物 WDTZ-08 4.65×1078 0.099 0 0.764 6** WDTZ-09 4.71×1069 0.087 2 0.700 2** 大陇乡湖积物 WDTZ-10 9.19×1025 0.034 5 0.871 3** WDTZ-11 2.25×1021 0.030 3 0.869 3** WDTZ-25 3.00×1022 0.031 8 0.922 5** 表 4 K和Vb与土壤理化性质之间的Pearson相关系数
Table 4. Pearson correlation coefficient between K, Vb and soil physical and chemical properties
参数 pH 有机质(%) Cd(mg·kg-1) 物理性粘粒(%) 等温吸附常数K 0.911** -0.595 -0.661 -0.721* 平衡吸附速率Vb 0.858** -0.370 -0.525 -0.717* -
Ahamada, K.U., Singh, R., Baruah, T., et al., 2018. Equilibrium and Kinetics Modeling of Fluoride Adsorption onto Activated Alumina, Alum and Brick Powder. Groundwater for Sustainable Development, 7: 452-458. https://doi.org/10.1016/j.gsd.2018.06.005 Aşçı, Y., Nurbaş, M., Açıkel, Y.S., 2008. A Comparative Study for the Sorption of Cd(II) by Soils with Different Clay Contents and Mineralogy and the Recovery of Cd(II) Using Rhamnolipid Biosurfactant. Journal of Hazardous Materials, 154(1-3): 663-673. https://doi.org/10.1016/j.jhazmat.2007.10.078 Bai, J., Ye, X., Jia, J., et al., 2017. Phosphorus Sorption-Desorption and Effects of Temperature, pH and Salinity on Phosphorus Sorption in Marsh Soils from Coastal Wetlands with Different Flooding Conditions. Chemosphere, 188: 677-688. https://doi.org/10.1016/j.chemosphere.2017.08.117 Cerqueira, B., Covelo, E.F., Andrade, L., et al., 2011. The Influence of Soil Properties on the Individual and Competitive Sorption and Desorption of Cu and Cd. Geoderma, 162(1): 20-26. https://doi.org/10.1016/j.geoderma.2010.08.013 Chen, S., Sun, T.H., Sun, L.N., et al., 2007. Sorption-Desorption Behavior of Cd2+ and Pb2+ in Rhizosphere and Bulk Soil. Environmental Sciences, 28(4): 4843-4851 (in Chinese with English abstract). http://www.ncbi.nlm.nih.gov/pubmed/17639948 Chen, D., Wang, X., Wang, X., et al., 2020. The Mechanism of Cadmium Sorption by Sulphur-Modified Wheat Straw Biochar and Its Application Cadmium-Contaminated Soil. The Science of the Total Environment, 714: 136550. https://doi.org/10.1016/j.scitotenv.2020.136550 Covelo, E.F., Andrade, M.L., Vega, F.A., 2004. Heavy Metal Adsorption by Humic Umbrisols: Selectivity Sequences and Competitive Sorption Kinetics. Journal of Colloid and Interface Science, 280(1): 1-8. https://doi.org/10.1016/j.jcis.2004.07.024 Elbana, T.A., Selim, H.M., 2010. Cadmium Transport in Alkaline and Acidic Soils: Miscible Displacement Experiments. Soil Science Society of America Journal, 74(6): 1956-1966. https://doi.org/10.2136/sssaj2010.0146 He, M.Y., Zheng, H.B., Huang, X.T., et al., 2011. Clay Mineral Assemblages in the Yangtze Drainage and Provenance Implications. Acta Sedimentologica Sinica, 29(3): 544-551(in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-CJXB201103017.htm Huang, S., Zhang, R.D., Zhang, J.Y., et al., 2012. Effects of Physical and Chemical Properties of Soil on Adsorption of Heavy Metals to Cadmium. Journal of Irrigation and Drainage, 31(1): 19-22(in Chinese with English abstract). Huang, Y.Y., Liu, D.D., Li, G.R., 2012. Adsorption Kinetics of As (Ⅲ) from Groundwater by Nanoscale Zero-Valent Iron. Earth Science, 37(2): 294-300(in Chinese with English abstract). http://www.mendeley.com/research/adsorption-kinetics-iii-groundwater-nanoscale-zerovalent-iron/ Inyang, H.I., Onwawoma, A., Bae, S., 2016. The Elovich Equation as a Predictor of Lead and Cadmium Sorption Rates on Contaminant Barrier Minerals. Soil and Tillage Research, 155: 124-132. https://doi.org/10.1016/j.still.2015.07.013 Itami, K., Yanai, J.T., 2006. Sorption and Desorption Properties of Cadmium and Copper on Soil Clays in Relation to Charge Characteristics. Soil Science and Plant Nutrition, 52(1): 5-12. https://doi.org/10.1111/j.1747-0765.2006.00015.x Ji, L.M., Qiu, J.L., Zhang, T.W., et al., 2012. Experiments on Methane Adsorption of Common Clay Minerals in Shale. Earth Science, 32(5): 1043-1050 (in Chinese with English abstract). http://d.wanfangdata.com.cn/Periodical/dqkx201205016 Krishnamurti, G.S.R., Naidu, R., 2003. Solid-Solution Equilibria of Cadmium in Soils. Geoderma, 113(1-2): 17-30. https://doi.org/10.1016/s0016-7061(02)00313-0 Lair, G.J., Gerzabek, M.H., Haberhauer, G., et al., 2006. Response of the Sorption Behavior of Cu, Cd, and Zn to Different Soil Management. Journal of Plant Nutrition and Soil Science, 169(1): 60-68. https://doi.org/10.1002/jpln.200521752 Li, C.F., Li, T.X., Zhang, X.Z., et al., 2017. Stabilization Characteristics of Cadmium in Some Typical Agricultures Oils. Journal of Agro-Environment Science, 36(1): 85-92 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-NHBH201701011.htm Li, P., Lang, M., Wang, X., et al., 2016. Sorption and Desorption of Copper and Cadmium in a Contaminated Soil Affected by Soil Amendments. Clean-Soil, Air, Water, 44(11): 1547-1556. https://doi.org/10.1002/clen.201500555 Lin, D.S., Xu, Y.M., Sun, G.H., et al., 2007. Effects of pH, Organic Matter and Hydrous Oxides on Competitive Adsorption of Cd2+ and Pb2+ by Soil. Journal of Agro-Environment Science, 26(2): 510-515(in Chinese with English abstract). http://www.cnki.com.cn/Article/CJFDTotal-NHBH200702019.htm Lin, Q., Xu, S.H., 2008. A Review on Competitive Adsorption of Heavy Metals in Soils. Soils, 40(5): 706-711(in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-TURA200805007.htm Liu, Y.Z., Xiao, T.F., Ning, Z.P., et al., 2013. Cadmium and Selected Heavy Metals in Soils of Jianping Area in Wushan County, the Three Gorges Region: Distribution and Source Recognition. Chinese Journal of Environmental Science, 34(6): 2390-2398(in Chinese with English abstract). http://www.researchgate.net/publication/255956196_Cadmium_and_selected_heavy_metals_in_soils_of_Jianping_area_in_Wushan_County_the_Three_Gorges_Region_Distribution_and_source_recognition Ma, P.G., Zhang, H.T., Ding, W.M., 2019. Kinetics and Thermodynamics of Modified Granular Activated Alumina for Fluoride Removal. Journal of Beijing University of Chemical Technology (Natural Science), 46(3): 1-6(in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-BJHY201903001.htm Manjuladevi, M., Anitha, R., Manonmani, S., 2018. Kinetic Study on Adsorption of Cr(VI), Ni(II), Cd(II) and Pb(II) Ions from Aqueous Solutions Using Activated Carbon Prepared from Cucumis Melo Peel. Applied Water Science, 8(1): 1-8. https://doi.org/10.1007/s13201-018-0674-1 Meng, F.D., Jiang, X., Jin, X.C., 2004. Physical-Chemical Characteristics of the Sediments in Lakes from the Middle and Lower Reaches of the Yangtze River. Research of Environmental Sciences, 17: 24-29(in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-HJKX2004S1006.htm Naidu, R., Kookana, R.S., Sumner, M.E., 1997. Cadmium Sorption and Transport in Variable Charge Soils: A Review. Journal of Environmental Quality, 26(3): 602- 617. https://doi.org/10.2134/jeq1997.00472425002600030004x Ren, B.Z., Wu, Y., Deng, D.P., et al., 2020. Effect of Multiple Factors on the Adsorption of Cd in an Alluvial Soil from Xiba, China. Journal of Contaminant Hydrology, 232: 103605. https://doi.org/10.1016/j.jconhyd.2020.103605 Satish, P., Sameer, R., Naseema, P., 2011. Removal of Methylene Blue, a Basic Dye from Aqueous Solutions by Adsorption Using Teak Tree (Tectona Grandis) Bark Powder. International Journal of Environmental Sciences, 5(1): 711-725. http://www.researchgate.net/publication/284971987_Removal_of_methylene_blue_a_basic_dye_from_aqueous_solutions_by_adsorption_using_teak_tree_Tectona_grandis_bark_powder Sauvé, S., Manna, S., Turmel, M. C., et al., 2003. Solid–Solution Partitioning of Cd, Cu, Ni, Pb, and Zn in the Organic Horizons of a Forest Soil. Environmental Science & Technology, 37(22): 5191-5196. https://doi.org/10.1021/es030059g Shirvani, M., Kalbasi, M., Shariatmadari, H., et al., 2006. Sorption–Desorption of Cadmium in Aqueous Palygorskite, Sepiolite, and Calcite Suspensions: Isotherm Hysteresis. Chemosphere, 65(11): 2178-2184. https://doi.org/10.1016/j.chemosphere.2006.06.002 Tang, H.X., Xue, H.B., Lin, G.Z., et al., 1981. Basic Characteristics of Adsorption of Cd Contaminants by Clay Minerals. Acta Scuentiae Circumstantiae, 1(2): 140-154(in Chinese with English abstract). Wang, H.M., Wang, B.G., Jin, M.G., et al., 2018. Spatial Distribution and Source of Heavy Metals in Alluvial Soils and Lacustrine Soils—A Case Study in Dangtu County, Anhui. Safety and Environmental Engineering, 25(5): 55-63(in Chinese with English abstract). http://search.cnki.net/down/default.aspx?filename=KTAQ201805009&dbcode=CJFD&year=2018&dflag=pdfdown Wang, L., 2010. Study of the Environmental Geochemical Behavior of Cd and Other Heavy Metals in the Changjiang River and Typical Soil in It's Basin(Dissertation). Chinese Academy of Geological Sciences, Beijing, 4-60(in Chinese with English abstract). Wang, Y.J., Zhou, D.M., Sun, R.J., et al., 2006. Competitive Adsorption Kinetics of Copper and Lead Ions in Soils. China Environmental Sciences, 26(5): 555-559(in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZGHJ200605010.htm Wang, Z., Zhang, L., Dang, X.L., 2012. Effect of the Freezing-Thawing on Kinetics of Adsorption-Desorption of the Soil Cadmium. Acta Scientiae Circumstantiae, 32(3): 721-725(in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-HJXX201203030.htm Xu, M.G., Li, J.M., Zhang, Q., 2004. Effect of pH on the Desorption Characteristics of Heavy Metals in Yellow Brown Soil. Ecology and Environment, 13(3): 312-315(in Chinese with English abstract). http://d.wanfangdata.com.cn/Periodical/tryhj200403004 Zhang, D.L., Jin, M.G., Fang, Y., et al., 2014. Adsorption and Desorption of Cadmium in Silty Loam under Acidic Conditions. Chinese Soil and Fertilizer, (4): 29-34(in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTotal-TRFL201404008.htm Zhang, Z.Q., Zhang, Y.P., Zhu, Z.H., 2000. Study on the Characteristics of Kinetic of Cadmium Retention on Soils. Acta Scientiae Circumstantiae, 20(3): 370-375(in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-HJXX200003022.htm Zhou, Y.T., Sherpa, S., Mcbride, M.B., 2020. Pb and Cd Chemisorption by Acid Mineral Soils with Variable Mn and Organic Matter Contents. Geoderma, 368: 114274. https://doi.org/10.1016/j.geoderma.2020.114274 Zong, L.G., Xu, X.Y., 2003. Advances in Adsorption and Desorption of Soil Cadmium. Ecology and Environment, 12(3): 331-335 (in Chinese with English abstract). 陈苏, 孙铁珩, 孙丽娜, 等, 2007. Cd2+、Pb2+在根际和非根际土壤中的吸附-解吸行为. 环境科学, 28(4): 4843-4851. https://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ200704027.htm 何梦颖, 郑洪波, 黄湘通, 等, 2011. 长江流域沉积物黏土矿物组合特征及物源指示意义. 沉积学报, 29(3): 544-551. https://www.cnki.com.cn/Article/CJFDTOTAL-CJXB201103017.htm 黄爽, 张仁铎, 张家应, 等, 2012. 土壤理化性质对吸附重金属镉的影响. 灌溉排水学报, 31(1): 19-22. https://www.cnki.com.cn/Article/CJFDTOTAL-GGPS201201005.htm 黄园英, 刘丹丹, 李桂荣, 2012. 纳米铁对地下水中As(Ⅲ)的吸附动力学. 地球科学, 37(2): 294-300. https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX201202015.htm 吉利明, 邱军利, 张同伟, 等, 2012. 泥页岩主要黏土矿物组分甲烷吸附实验. 地球科学, 32(5): 1043-1050. http://www.earth-science.net/article/id/2308 李传飞, 李廷轩, 张锡洲, 等, 2017. 外源镉在几种典型农耕土壤中的稳定化特征. 农业环境科学学报, 36(1): 85-92. https://www.cnki.com.cn/Article/CJFDTOTAL-NHBH201701011.htm 林大松, 徐应明, 孙国红, 等, 2007. 土壤pH、有机质和含水氧化物对镉、铅竞争吸附的影响. 农业环境科学学报, 26(2): 510-515. doi: 10.3321/j.issn:1672-2043.2007.02.020 林青, 徐绍辉, 2008. 土壤中重金属离子竞争吸附的研究进展. 土壤, 40(5): 706-711. doi: 10.3321/j.issn:0253-9829.2008.05.005 刘意章, 肖唐付, 宁增平, 等, 2013. 三峡库区巫山建坪地区土壤镉等重金属分布特征及来源研究. 环境科学, 34(6): 2390-2398. https://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ201306051.htm 马培根, 张海涛, 丁文明, 2019. 颗粒改性活性氧化铝吸附除氟动力学和热力学的研究. 北京化工大学学报(自然科学版), 46(3): 1-6. https://www.cnki.com.cn/Article/CJFDTOTAL-BJHY201903001.htm 孟凡德, 姜霞, 金相灿, 2004. 长江中下游湖泊沉积物理化性质研究. 环境科学研究, 17: 24-29. https://www.cnki.com.cn/Article/CJFDTOTAL-HJKX2004S1006.htm 汤鸿霄, 薛含斌, 林国珍, 等, 1981. 粘土矿物吸附锦污染物的基本特征. 环境科学学报, 1(2): 140-154. https://www.cnki.com.cn/Article/CJFDTOTAL-HJXX198102003.htm 王慧敏, 汪丙国, 靳孟贵, 等, 2018. 冲积与湖积成因土壤重金属的空间分布特征及其来源——以安徽当涂县为例. 安全与环境工程, 25(5): 55-63. https://www.cnki.com.cn/Article/CJFDTOTAL-KTAQ201805009.htm 王岚, 2010. 长江水系及流域典型土壤中Cd等重金属元素的环境地球化学行为研究(博士学位论文). 北京: 中国地质科学院, 4-60. 王玉军, 周东美, 孙瑞娟, 等, 2006. 土壤中铜、铅离子的竞争吸附动力学. 中国环境科学, 26(5): 555-559. doi: 10.3321/j.issn:1000-6923.2006.05.011 王展, 张良, 党秀丽, 等, 2012. 冻融作用对土壤镉动力学吸附解吸的影响. 环境科学学报, 32(3): 721-725. https://www.cnki.com.cn/Article/CJFDTOTAL-HJXX201203030.htm 徐明岗, 李菊梅, 张青, 2004. pH对黄棕壤重金属解吸特征的影响. 生态环境, 13(3): 312-315. doi: 10.3969/j.issn.1674-5906.2004.03.004 张德乐, 靳孟贵, 方远, 等, 2014. 酸性条件下粉砂质壤土对Cd~(2+)的吸附解吸特性研究. 中国土壤与肥料, (4): 29-34. https://www.cnki.com.cn/Article/CJFDTOTAL-TRFL201404008.htm 张增强, 张一平, 朱兆华, 2000. 镉在土壤中吸持的动力学特征研究. 环境科学学报, 20(3): 370-375. doi: 10.3321/j.issn:0253-2468.2000.03.025 宗良纲, 徐晓炎, 2003. 土壤中镉的吸附解吸研究进展. 生态环境, 12(3): 331-335. doi: 10.3969/j.issn.1674-5906.2003.03.019 -










 下载:
下载: