Zinc Isotope Geochemistry of Marine Sediments and Its Applications: A Review
-
摘要: 锌同位素体系是海洋地球化学研究的新示踪剂,应用于示踪海水中锌元素的来源及其运移过程.海洋沉积物作为锌元素重要的"源"与/或"汇",其锌同位素组成的研究有助于理解海洋锌元素的地球化学循环.海洋沉积物记录了海水组成的信息,可以反演古海水锌同位素组成的变化,前提是理解沉积物与海水之间的分馏.对海水及海洋不同储库锌同位素研究进行系统总结,包括河流输入、热液体系、不同类型海洋沉积物(如富碳酸盐的沉积物、陆源硅酸盐碎屑、硅质沉积物、铁锰结核、贫氧-缺氧沉积物)的锌同位素组成,阐述了海洋沉积物锌同位素组成变化在古气候、古环境重建以及古海洋学等领域的应用以及重要性.Abstract: Zinc isotope represents new tracers of oceanography, which is used to trace the source and migration of zinc in seawater. As important sources and/or sinks of zinc, the study of zinc isotopic composition of marine sediments is of great significance to our understanding of geochemical cycling of zinc. In addition, marine sediments record the composition of seawater, and the study of zinc isotope fractionation between marine sediments and seawater is a prerequisite for tracing zinc isotopic composition of ancient seawater. In this paper, researches on zinc isotopes of seawater and reservoirs in the ocean are systematically summarized, including zinc isotope study of riverine inputs, hydrothermal systems and different types of marine sediments (carbonate-rich sediments, terrigenous silicate fragments, siliceous sediments, ferromanganese nodules and suboxic/anoxic sediments), to explain the applications and importance of zinc isotope variations of marine sediments in paleoclimate, paleoenvironment and paleoceanography.
-
Key words:
- marine sediment /
- Zn isotope /
- marine geochemical cycling /
- paleoclimate /
- geochemistry
-
锌(Zn)原子序数为30,平均原子量为65.38,位于元素周期表第四周期、第二副族,是与人类活动密切相关的金属元素,具有中度挥发性、亲石性和亲铜性(亲石性大于亲铜性)(王照雪等,2020).锌原子电子构型为Zn0([Ar]3d104s2),它容易失去最外层的两个电子,形成铜型离子稳定结构(Zn2+([Ar]3d10))(Moynier et al., 2017).因此,在化合物或复合离子中,主要以Zn2+形式存在(Moynier et al., 2017).锌是生命活动必需的微量营养元素,它在浮游植物中的丰度仅次于铁,在硅藻中甚至高于铁(Twining and Baines, 2013;Morel et al., 2014).它不仅在DNA复制和转录中具有重要作用,而且在碳酸氢酶和碱性磷酸酶中起着辅助因子的作用(Sinoir et al., 2012).在海洋中,锌含量和硅含量具有明显的耦合关系,在表层水体中含量较低,而在深海水体中,二者含量均达到最大值(Bruland,1980;Martin et al., 1989;Zhao et al., 2014;Vance et al., 2017).
锌有5个稳定同位素:64Zn、66Zn、67Zn、68Zn、70Zn,其丰度分别为49.2%、27.8%、4.0%、18.4%、0.6%(Shields et al., 1964).锌同位素组成一般用δXZn(‰)=[(XZn/64Zn)样品/(XZn/64Zn)标准)-1]× 1 000表示,其中X = 66、67、68或70,常用δ66Zn表示,目前一般选用JMC 3-0749L Zn作为锌的参考标准(Maréchal et al., 2000).由于该标样已经停产,IRMM-3702和NIST SRM 683等被推荐作为新的国际标准,它们相对于JMC 3-0749L的值分别为+0.27 ± 0.03‰(Wang et al., 2017)和+0.12 ± 0.04‰(Chen et al., 2015;Yang et al., 2018).
海洋沉积物作为锌元素海洋地球化学循环中重要的“源”与“汇”,其同位素组成对于我们理解锌元素参与的海洋生物化学和地球化学循环有重要意义.例如,Little et al.(2016)根据海洋锌的同位素质量平衡,发现富有机质的贫氧大陆边缘沉积物是海水中轻锌的重要“汇”,其通量和铁锰氧化物中的重锌相当.此外,海水的锌同位素变化可以为古环境和古气候变化提供有价值的线索,而沉积物可能记录了海水组成的信息,理解其同位素组成的受控因素是研究古海洋记录的前提与关键.本文总结了海水及海洋不同储库的锌同位素组成,包括河流输入、热液系统以及不同类型海洋沉积物的锌同位素组成(如碳酸盐沉积物、陆源硅酸盐碎屑、硅质沉积物、铁锰氧化物以及贫氧-缺氧沉积物),综述海洋沉积物的锌同位素地球化学在古气候、古环境重建以及古海洋学等领域的应用、研究意义以及重要性.
1. 现代海水锌同位素组成
在生物界中,锌具有重要的结构功能,通常作为生物生长所需要酶的辅助因子的成分,存在于100种特异性酶中(United States National Research Council,2000).它在海洋学领域的意义主要是在碳酸氢酶(参与固碳)和碱性磷酸酶(在磷的吸收和循环中起活性作用)两种关键酶中作为辅酶(Sinoir et al., 2012).实验表明,锌元素对于维持浮游植物的生长和控制海洋初级生产力具有重要作用,并在一定程度上影响浮游植物群落的组成(Brand et al., 1983;Shaked et al., 2006;John et al., 2007;Köbberich and Vance, 2017).例如,在高营养盐低叶绿素(HNLC)海区中,大范围缺锌可能会影响浮游生物的生长和生物群落结构(Morel et al., 1994).海水中锌元素的垂直循环与多个过程有关,包括表层生物吸收、颗粒物沉降、颗粒物分解、上升流活动和沉积物攫取,其垂直分布趋势与硝酸盐、磷酸盐和硅酸盐类似(Bruland,1980;Martin et al., 1989),主要表现为“表层亏损,深部富集”.例如,在开放的太平洋中,深层海水锌的含量大约为表层海水的250倍(Lohan et al., 2002).
现有的研究表明,表层海水锌同位素组成变化范围较大(变化高达2.0‰,从-1.1‰到+0.9‰,Conway and John, 2015),而深海则极其均一(δ66Zn = +0.5‰,Bermin et al., 2006;Boyle et al., 2012;Conway and John, 2014,2015;Zhao et al., 2014;Samanta et al., 2017;John et al., 2018;Vance et al., 2019).在表层海水中,由于浮游生物优先吸收或摄取同位素较轻的锌,而使周围环境海水具有相对较重的锌同位素组成(Bermin et al., 2006;John et al., 2007).而表层海水的下沉有机颗粒对锌的络合吸附过程则倾向富集重锌同位素,导致海水相对富集较轻的锌同位素(Conway and John, 2014).并且随着浮游生物死亡解体,细胞成分中轻的锌同位素可以重新释放到海水中,海水锌同位素回到“原始值”(Conway and John, 2014;Zhao et al., 2014).此外,锌的硫化物沉淀也可以萃取溶液中轻的锌同位素(Little et al., 2016;Vance et al., 2016;Liang et al., 2020),造成表层海水锌同位素升高.
2. 海洋不同储库的锌同位素研究
2.1 海洋中不同来源锌的同位素组成
2.1.1 河流输入
河流输入作为痕量元素输入海洋的主要途径,也是海洋中溶解锌的重要来源之一.Little et al.(2014a)报道了全球主要入海河流的锌同位素组成,δ66Zn从-0.12‰到+0.88‰变化,平均组成约为+0.33‰,与陆壳平均值组成(+0.27 ± 0.07‰,1SD,Little et al., 2016)接近,明显低于海水平均值(+0.5‰).计算表明河流向海洋输入锌的通量大约为5.9×108 mol/a(Little et al., 2014a).
现代河水的锌同位素组成会受到人类活动的显著影响.Chen et al.(2008)对塞纳河流域锌含量及其同位素的研究表明,塞纳河及其支流的溶解锌含量从上游到下游呈现逐渐增加的趋势(1~74 nmol/L),而δ66Zn从源头到河口呈现逐渐降低的趋势(+0.58‰~+0.07‰).这是当地金属运河管道污染、碳酸盐岩溶解以及生活污水共同作用的结果,说明锌同位素在示踪河流重金属污染方面具有很大的潜力.然而,河流的锌同位素组成受到多种自然因素控制,例如矿物吸附过程发生的同位素分馏有时会掩盖污染源的锌同位素特征,所以利用锌同位素示踪污染锌的来源,前提是需要解析河流锌同位素组成的受控因素(Liu et al., 2020;Zimmermann et al., 2020).
2.1.2 其他输入
海底热液作为海洋溶解锌一个重要的输入端元,目前报道的同位素变化范围较大(-0.5‰~+0.24‰).高温水-岩相互作用过程可以使热液优先富集重的锌同位素,因为硫化物连续批式淋滤实验表明在淋滤的初始阶段,流体富集重锌同位素(Fernandez and Borrok, 2009),此外富集轻锌同位素的硫化物先沉淀也可导致流体富集重同位素(王跃和朱祥坤,2010).东太平洋隆(EPR)海底热液通道、大西洋中脊的TAG热液场和Guaymas盆地内热液的锌同位素组成,其质量加权平均值为+0.24‰(John et al., 2008);而瓜德罗普岛活火山热水的质量加权平均锌同位素组成为+0.63‰,高于已知的输入端元和海水的锌同位素组成(Chen et al., 2014).此外,根据热液柱的实测值推算大西洋中脊TAG热液场的δ66Zn值约为-0.5‰(Conway and John, 2014),远低于其他报道过的火山热液及海底热液值.热液的平均锌同位素组成和分馏机制还未明确,难以根据同位素组成来估计通量,需进一步的调查研究.
此外,在现代海洋中,大气沉降也是重要的海洋锌来源之一,其锌同位素组成约为+0.37‰(Little et al., 2014a),其中锌的主要来源和人类活动有关.
2.2 海洋沉积物的锌同位素组成特征
2.2.1 碳酸盐岩锌同位素组成
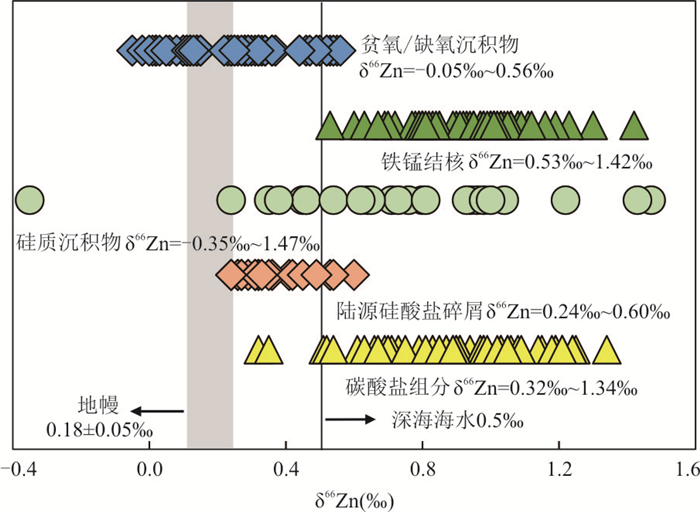
Maréchal et al.(2000)最早发现富碳酸盐沉积物(平均δ 66Zn = +0.74‰,N = 2)的锌同位素组成明显高于其他海洋沉积物.Pichat et al.(2003)对17.5万年以来赤道地区太平洋东部岩心的碳酸盐组分的分析结果表明海洋沉积物中碳酸盐组分的δ 66Zn变化范围较大,为+0.32‰~+1.34‰,均高于地壳值(+0.27 ± 0.07‰,1SD,Little et al., 2016).这与实验研究结果一致,锌在碳酸盐表面的吸附和沉淀过程中,重锌同位素优先与碳酸盐结合(Dong and Wasylenki, 2016;Mavromatis et al., 2019).Zhao et al.(2021)测定了巴哈马群岛、巴拿马以及波斯湾的海水、沉积物以及浅水沉积的碳酸盐剖面的锌同位素.结果表明,以文石鲕粒和球粒为主的碳酸盐样品δ 66Zn (-0.56‰~+0.30‰;大部分为0.00‰~+0.30‰)与海水δ 66Zn(+0.06‰~+0.24‰)重叠,而碳酸盐泥δ 66Zn(+0.37‰~+0.55‰)高于海水值.另外,除了海胆骨骼中高Mg方解石相对于海水富集重锌以外,其余来自叠层石、双壳类、绿藻等生物中文石的δ66Zn(+0.03‰~+0.39‰)均与海水没有明显差别,说明生物骨骼中,文石质碳酸盐(如多细胞生物或藻类)以及微生物文石(如叠层石)具有示踪海水锌同位素组成变化的潜力(Zhao et al., 2021).
2.2.2 陆源硅酸盐碎屑锌同位素组成
海洋沉积物中的陆源硅酸盐碎屑组分的Zn同位素组成与地壳类似.Bentahila et al.(2008)对台湾5个海洋岩心的沉积物进行了硅酸盐组分的提取,首次报道了海洋沉积物中硅酸盐组分的锌同位素组成.这些沉积物硅酸盐碎屑组分的δ 66Zn为+0.24‰~+0.6‰(平均值为+0.36 ± 0.21‰,N = 21,2SD),除了来自岩心MD9271-45以及VM33-95的样品显示出较高的δ66Zn以外(+0.46‰~+0.56‰),其余沉积物与上地壳有相似的δ66Zn值.富集重锌的硅酸盐碎屑组分可能继承了源区的特征,其同位素组成与源区的基岩以及源区发育的卑南溪的颗粒相似.
硅酸盐矿物风化能为生物提供所必需的营养成分,而硅酸盐碎屑作为沉积物组分中的重要组成部分,其锌同位素组成保存了丰富的陆源输入的信息.有关沉积物中硅酸盐组分锌同位素的报道仍然比较缺乏,后续研究有待进一步完善不同储库的锌同位素组成以及硅酸盐锌同位素在地球科学中的应用.
2.2.3 硅质沉积物的锌同位素组成
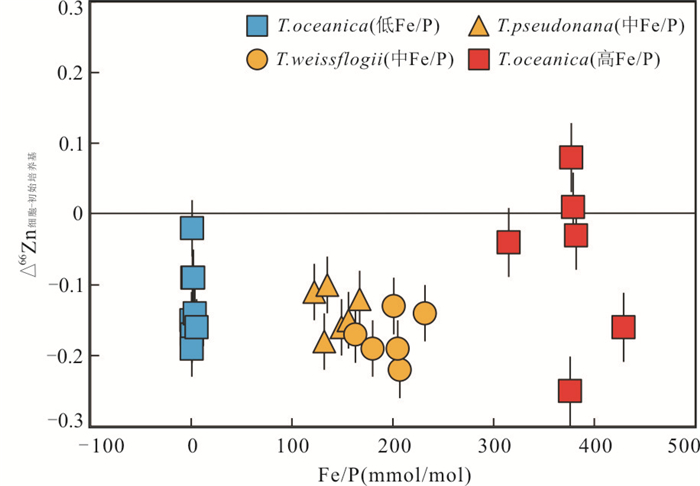
硅质沉积物中生物成因锌的富集主要来源于硅藻蛋白、海绵、硅藻细胞等硅质生物.锌是碳酸氢酶(参与固碳)的重要组成成分(Sinoir et al., 2012),而硅藻参与了地球上超过20%的碳的固定(Armbrust,2009),其生理结构与锌的生物可利用度密切相关(Anderson et al., 1978;Harrison and Morel, 1986;Sunda and Huntsman, 1992),不同环境下硅藻细胞有不同的锌同位素分馏行为,对于理解锌元素参与的海洋生物化学循环具有重要意义.硅藻表面富集重的锌同位素(Gélabert et al., 2006),这是由于吸附过程中锌从六配位的水合物(Zn(H2O)6)变为四配位的羧基、氨基和羟基的络合物(Pokrovsky et al., 2005).John et al.(2007)对T. Oceanica硅藻培养实验表明,培养基中Zn2+的浓度决定了T. Oceanica吸收Zn的主要方式,当培养基中Zn2+浓度较高时以低亲合转运为主,Zn2+浓度较低时则相反,且两种方式的Zn同位素分馏相差较大(Δ66Zn细胞-培养基(高亲合转运) = -0.2‰;Δ66Zn细胞-培养基(低亲合转运) = -0.8‰).Köbberich and Vance(2017)在恒定的Zn浓度和不同的Fe浓度的培养基条件下,对T. Oceanica和Chaetoceros sp.两种类型的硅藻进行了一系列培养实验,发现Fe的限制不仅控制着硅藻的生长,还影响着其对Zn的摄取.T. Oceanica在不同Fe浓度下仍可以保持较高的生长速率以及Zn的摄取速率,且摄取的Zn与培养基中游离的Zn2+之间仅有较小的Zn同位素分馏(Δ66Zn硅藻-培养基=-0.02‰~-0.19‰).然而,Chaetoceros在低铁浓度下,Zn吸收速率低,且具有重于培养基0.85‰的锌同位素特征.这可能是由于在低铁水平下,细胞外聚合物质的释放优先络合重锌,或者吸收速率足够慢,重Zn同位素形成更稳定的化合物导致的.这一发现对于解释表层海水中较轻的Zn同位素组成提供了新的见解,即表层海水中较轻的Zn同位素组成可能是在极低Zn的摄取速率条件下,由硅藻细胞优先吸收重Zn同位素所驱动的. Köbberich and Vance (2018)进一步进行培养实验,以评估Zn同位素吸附到海洋硅藻表面Fe氢氧化物过程中的分馏.T. oceanica在高Fe/P时,细胞表面会形成Fe的氢氧化物,重锌同位素会被优先吸附到Fe的氢氧化物上,从而掩盖部分生物吸收轻Zn的信号.而在细胞内相对较低或中等Fe/P比值条件下,氢氧化物吸附表现出的锌同位素分馏并不明显(Δ66Zn细胞-初始培养基 < 0,图 1)(Köbberich and Vance, 2018),与Köbberich and Vance(2017)中T. oceanica的结果一致.
 图 1 不同种属硅藻细胞与初始培养基的Zn同位素分馏值与细胞内Fe/P比值关系数据来源于Köbberich and Vance(2017, 2018)Fig. 1. Zn isotope fractionation between biomass and the initial culturing medium (Δ66Zn), as observed for three different Thalassiosira strains with different Fe/P ratios
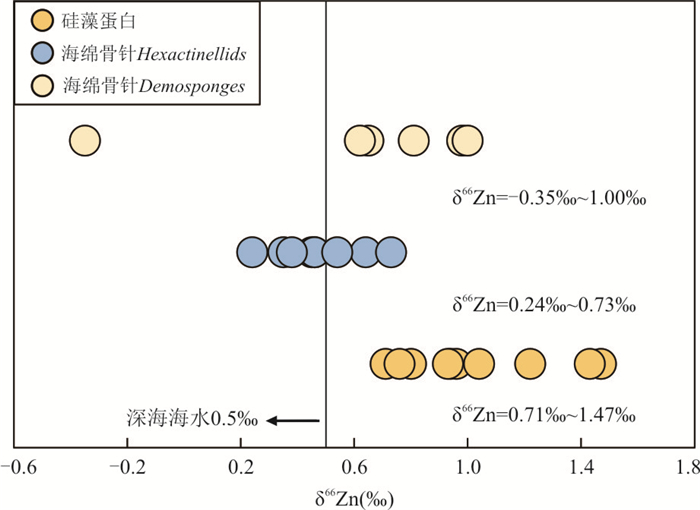
图 1 不同种属硅藻细胞与初始培养基的Zn同位素分馏值与细胞内Fe/P比值关系数据来源于Köbberich and Vance(2017, 2018)Fig. 1. Zn isotope fractionation between biomass and the initial culturing medium (Δ66Zn), as observed for three different Thalassiosira strains with different Fe/P ratiosAndersen et al.(2011)从南大洋全新世的沉积物中提取了硅藻蛋白并测定了其中锌的含量和Zn同位素组成.硅藻蛋白中Zn的浓度为1~14 μg/g,δ66Zn为+0.76‰~+1.47‰,平均值为+1.03±0.53‰(N = 10,2SD),明显重于现代海水的δ 66Zn (+0.5‰),这与生物优先吸收利用较轻的Zn同位素相反.硅藻蛋白中Zn同位素偏重的原因可能是(1)表层海水中游离Zn2+(被摄取进入蛋白石中)与配体结合态Zn之间存在平衡分馏,导致硅藻蛋白中较重的Zn同位素组成;(2)细胞外的Zn被摄取进入硅藻蛋白的过程中发生分馏;(3)硅藻蛋白记录了已经受到生物活动影响后的海水的δ66Zn.此外,Zn/Si与δ66Zn之间存在较好的负相关关系,当δ66Zn最低时,Zn/Si具有最大值,且蛋白石埋藏速率最高. Ellwood and van der Berg(2000)的培养实验表明,尽管硅藻中大于97%的Zn都赋存于有机物中,但蛋白石中Zn/Si与生长介质中游离的Zn2+可利用度成正相关关系.表层海水中Zn的亏损一般归因于硅藻或其他浮游植物细胞的摄取,而Zn/Si的系统变化可能反映了硅藻蛋白形成时周围表层海水中生物可利用Zn的浓度,即随着表层水体逐渐远离上升流区域,Zn的生物利用率增加,导致残余海水亏损较轻的Zn,而δ 66Zn变重(Andersen et al., 2011).此外,通过Zn/Si与δ 66Zn的相关关系所推算的Zn2+浓度以及锌同位素分馏系数也与前人研究相吻合(Andersen et al., 2011),说明硅藻蛋白很有可能记录下了它所形成时表层海水的锌同位素组成的变化,是研究海洋锌的生物地球化学循环的潜在示踪剂.
Hendry and Andersen(2013)研究了Hexactinellids和Demosponges两种海洋硅质海绵骨针的Zn同位素组成,显示出较大的δ 66Zn变化范围(-0.35‰~+1.04‰),比硅藻蛋白的变化范围更大(+0.71‰~+1.47‰,Andersen et al., 2011).两种海绵骨针的Zn同位素组成存在差异,其中Demosponges为-0.35‰~+1.04‰,Hexactinellids为+0.24‰~+0.73‰,这可能是由不同的摄食习性和内部生理结构造成的.Demosponges的δ 66Zn变化范围较大,可能是由于Demosponges通过吞噬作用摄食,能够吞噬粒径范围很大的颗粒,最高可以直接吞噬大于50 μm的颗粒(Reiswig,1971),不同颗粒物质有不同的Zn同位素组成,且摄取进入细胞的过程也会产生内部的Zn同位素分馏.对于Hexactinellids,虽然Zn含量有明显变化,但δ66Zn基本保持不变,与深海平均Zn同位素值相似(+0.5‰),这是由于Zn通过海绵开放的内部结构迁移,与海水之间无明显分馏,可以记录平均海水的锌同位素组成.
生物成因硅质沉积物的锌同位素组成变化较大(图 2),硅质生物所处的环境条件(例如环境中Zn2+、Fe浓度的不同)、生物所属种类的不同以及生长习性的差异都会影响其Zn同位素的组成,因此,在解释其代表的生长环境时,应考虑到铁的限制、Zn的吸收速率、有机络合程度等多种影响因素.另外,目前对于海洋硅藻等生物摄取Zn的同位素分馏的实验培养研究仍然比较缺乏,进一步的培养实验研究有待进行.
2.2.4 铁锰氧化沉积物的锌同位素组成
铁锰结核是一种以Fe、Mn元素为主且富含Ni、Co、Cu等金属元素的海底自生沉积矿物集合体(Hein et al., 2013),它广泛分布在全球大洋中,被认为是能够记录其形成过程中海洋环境信息的良好载体.作为海洋Zn元素另一重要的汇,Maréchal et al.(2000)和Little et al.(2014a, 2014b)的研究表明,铁锰结核的Zn同位素组成变化较大,为+0.53‰~+1.42‰,平均值+0.95 ± 0.33‰(N = 65,2SD),均高于现代海水平均值(+0.5‰),这是由于吸附Zn的过程中,配位数减小,优先吸附较重的66Zn(Fujii et al., 2010, 2011;Little et al., 2014b). Maréchal et al.(2000)最早对现代不同大洋的40个Fe-Mn结核外壳进行Zn同位素研究,发现其Zn同位素分布与地区呈现规律性变化,与纬度变化呈现正相关而与深度呈现负相关关系,可能受控于海洋初级生产力.在太平洋和大西洋中部,δ66Zn多以~+0.6‰~ +0.8‰为主,而在环南极洋流、太平洋北部和大西洋北部则多大于+1.0‰.部分大洋的表层受到生物优先摄取轻Zn的影响,富集重Zn同位素,但是随着海洋内部海水的交换(例如上升流),深部海水对表层海水的补给,表层海水64Zn的亏损减弱;然而在高纬度地区,温跃层阻断了深水对表层海水的补给,导致高纬度地区相对于低纬度地区的表层海水具有更重的Zn同位素组成.随着近年来进一步的研究,发现全球海水的δ66Zn的纵向分布和纬度分布受控于更多因素,尽管Maréchal et al.(2000)的最初研究表明铁锰结核可以作为记录海洋生产力变化的潜在指标,但铁锰结核中δ66Zn的变化机制仍有待进一步研究.
2.2.5 贫氧-缺氧沉积物的锌同位素组成
东北太平洋和东南太平洋边缘贫氧且富有机质的沉积物,具有轻于平均海水的δ 66Zn值(-0.05‰~+0.36‰,平均值为+0.15 ± 0.24‰,N = 36,2SD).Little et al.(2016)将这种较轻的Zn同位素组成特征归因于:(1)透光带中海洋浮游植物对Zn的摄取,优先吸收较轻的Zn同位素,并将这种信号保存在富有机质的沉积物中;(2)或是由于自生矿物(如ZnS)的沉淀,较轻的Zn同位素进入硫化物并被保存在沉积物中.Vance et al.(2016)研究发现在缺氧硫化的黑海盆地中,硫化物对轻Zn的萃取是造成水体Zn同位素组成偏重的原因.
贫氧-缺氧沉积物作为海洋轻Zn同位素一个重要的汇,解释了Zn同位素在海洋中的质量平衡.Little et al.(2014a, 2014b, 2016)通过对全球河水、大气沉降、Fe-Mn结核、沉积碳酸盐岩的Zn同位素研究发现,现代海洋中Zn的输入端元δ66Zn均轻于海水平均值(+0.5‰),但其输出端元δ66Zn(如铁锰结核、碳酸盐、硅质沉积物等)却明显高于平均海水Zn同位素组成,这不符合质量守恒.贫氧-缺氧沉积物的Zn同位素组成是海洋Zn同位素循环中重要的具有轻Zn同位素组成的输出端元,平衡了其他高于平均海水Zn同位素组成的“汇”(如铁锰结核、碳酸盐、硅质沉积物等).
3. 海洋沉积物锌同位素研究在古环境中的应用
作为生命活动必需的微量营养元素,海洋沉积物中锌元素及其同位素的生物及地球化学循环与海洋生物生产力密切相关,并且可以为研究过去海洋的环境变化和生物地球化学循环提供有价值的线索.已有的研究主要将锌同位素应用于两个方面:示踪海洋中突发大量的陆源锌输入和示踪锌在各类沉积物中的比例.
Liu et al.(2017)发现二叠纪末生物大灭绝前3.5万年地层中的碳酸盐岩的Zn同位素出现了高达0.5‰(从~0.83‰到~0.34‰)的负漂,火山灰、热液流体以及强烈风化的大火成岩省中大量轻Zn的快速输入可能是导致Zn同位素负漂的主要原因,为大规模火山作用是二叠纪生物灭绝的主要机制提供了重要依据.碳酸盐岩所记录的锌同位素负漂也在Cenomanian-Turonian大洋缺氧事件(Oceanic Anoxic Event 2,OAE 2)中发现2次,第1次由于大规模岩浆活动相关的陆源输入增加,第2次与OAE 2期间一次广泛的海底再氧化事件(The Plenus Cold Event)相关,此次再氧化事件过程可能诱发大型底栖有孔虫的灭绝(Sweere et al., 2018).
Pichat et al.(2003)首次发现17.5万年前至今的碳酸盐锌同位素变化与冰期-间冰期耦合,推测高δ66Zn记录了温跃层增强以及生产力升高.新元古代“雪球地球”后盖帽碳酸盐岩中的锌、镉和碳同位素升高也指示了冰期之后初级生产力的恢复,而冰期之后的锌同位素组成降低与硫化物埋藏有关(Kunzmann et al., 2013;John et al., 2017;Yan et al., 2019).同样,华南陈家园子陡山沱组一、二段的碳酸盐岩中的锌和碳同位素正漂事件,指示了约620 Ma的初级生产力升高以及有机碳埋藏增加,与古生物化石记录相互印证了一次生物繁盛事件(Lv et al., 2018).Wang et al.(2018)通过分析广西付合界限灰岩Zn-Sr同位素的协同变化,提出气候变冷导致的海平面下降是最终造成Frasnian-Famennian灭绝事件的因素.另外,缺氧且富有机质的沉积物也可以作为记录全球古海洋环境变化的潜在指标.Sweere et al.(2020)在晚白垩世摩洛哥塔尔法亚盆地沉积的富有机质的沉积物和英格兰南部同时期沉积的海相碳酸盐岩剖面中有相似的δ66Zn变化趋势,其δ66Zn以及δ114Cd的正漂与有机质广泛埋藏有关,而Plenus Cold Event前δ66Zn的降低与海底再氧化事件相关,降低了埋藏进入沉积物中轻Zn的比例或反映了已经进入沉积物中轻Zn的再矿化.上述研究表明,碳酸盐以及缺氧且富有机质的沉积物锌同位素组成可作为海洋环境的灵敏示踪剂,结合多指标可以反映火山事件带来的大量锌输入事件和初级生产力、有机埋藏等变化.
4. 结论与展望
本文总结了海水的锌同位素组成、海洋中不同锌的来源、不同类型海洋沉积物的锌同位素组成,其中重点阐述了海洋沉积物的锌同位素地球化学研究(图 3).锌对海洋生命具有重要意义,而锌同位素可作为海洋环境的灵敏示踪剂.海洋沉积物作为锌元素一个重要的汇,其锌同位素组成的研究对于我们理解现代海洋锌循环具有重要意义,且具有反映(古)海水的锌同位素组成变化的潜力.现有的研究表明,锌同位素可以示踪沉积物中锌的来源、海洋初级生产力的变化以及有机碳的埋藏等.对海洋沉积物的锌同位素组成进行重新评估和研究是将锌同位素体系作为古环境、古海洋重建指标的重要前提.目前,对海洋沉积物的Zn同位素研究还很少,海洋沉积物作为联系古环境、古海洋的媒介,需要更多地去探索,以完善锌同位素在不同地质储库中的分布(如硅质沉积物的Zn同位素组成)、了解锌同位素的地球化学循环过程(如海洋表层Zn同位素组成变化的关键控制机制)并推动锌同位素在古海洋、古环境、古气候以及其他地球科学领域的应用.
致谢: 感谢审稿人和编辑提出的宝贵意见和建议! 图 3 海洋沉积物的锌同位素组成统计数据来源于Maréchal et al.(2000);Pichat et al. (2003);Bermin et al. (2006);Andersen et al. (2011);Boyle et al. (2012);Hendry and Andersen (2013);Conway and John(2014, 2015);Little et al.(2014a, 2014b, 2016);Zhao et al. (2014);Vance et al.(2016, 2019);Samanta et al. (2017);Wang et al. (2017);John et al. (2018);McCoy-West et al. (2018);Sossi et al. (2018);Liu et al. (2019)Fig. 3. A compilation of Zn isotopic composition of marine sediments
图 3 海洋沉积物的锌同位素组成统计数据来源于Maréchal et al.(2000);Pichat et al. (2003);Bermin et al. (2006);Andersen et al. (2011);Boyle et al. (2012);Hendry and Andersen (2013);Conway and John(2014, 2015);Little et al.(2014a, 2014b, 2016);Zhao et al. (2014);Vance et al.(2016, 2019);Samanta et al. (2017);Wang et al. (2017);John et al. (2018);McCoy-West et al. (2018);Sossi et al. (2018);Liu et al. (2019)Fig. 3. A compilation of Zn isotopic composition of marine sediments -
图 1 不同种属硅藻细胞与初始培养基的Zn同位素分馏值与细胞内Fe/P比值关系
数据来源于Köbberich and Vance(2017, 2018)
Fig. 1. Zn isotope fractionation between biomass and the initial culturing medium (Δ66Zn), as observed for three different Thalassiosira strains with different Fe/P ratios
图 2 不同种类硅质沉积物的Zn同位素组成
数据来源于Andersen et al.(2011);Hendry and Andersen(2013)
Fig. 2. Zn isotopic composition of different types of siliceous sediments
图 3 海洋沉积物的锌同位素组成统计
数据来源于Maréchal et al.(2000);Pichat et al. (2003);Bermin et al. (2006);Andersen et al. (2011);Boyle et al. (2012);Hendry and Andersen (2013);Conway and John(2014, 2015);Little et al.(2014a, 2014b, 2016);Zhao et al. (2014);Vance et al.(2016, 2019);Samanta et al. (2017);Wang et al. (2017);John et al. (2018);McCoy-West et al. (2018);Sossi et al. (2018);Liu et al. (2019)
Fig. 3. A compilation of Zn isotopic composition of marine sediments
-
Andersen, M. B., Vance, D., Archer, C., et al., 2011. The Zn Abundance and Isotopic Composition of Diatom Frustules, a Proxy for Zn Availability in Ocean Surface Seawater. Earth and Planetary Science Letters, 301(1/2): 137-145. https://doi.org/10.1016/j.epsl.2010.10.032 Anderson, M. A., Morel, F. M. M., Guillard, R. R. L., 1978. Growth Limitation of a Coastal Diatom by Low Zinc Ion Activity. Nature, 276(5683): 70-71. https://doi.org/10.1038/276070a0 Armbrust, E. V., 2009. The Life of Diatoms in the World's Oceans. Nature, 459(7244): 185-192. https://doi.org/10.1038/nature08057 Bentahila, Y., Ben Othman, D., Luck, J. M., 2008. Strontium, Lead and Zinc Isotopes in Marine Cores as Tracers of Sedimentary Provenance: A Case Study around Taiwan Orogen. Chemical Geology, 248(1/2): 62-82. https://doi.org/10.1016/j.chemgeo.2007.10.024 Bermin, J., Vance, D., Archer, C., et al., 2006. The Determination of the Isotopic Composition of Cu and Zn in Seawater. Chemical Geology, 226(3/4): 280-297. https://doi.org/10.1016/j.chemgeo.2005.09.025 Boyle, E. A., John, S., Abouchami, W., et al., 2012. GEOTRACES IC1 (BATS) Contamination-Prone Trace Element Isotopes Cd, Fe, Pb, Zn, Cu, and Mo Intercalibration. Limnology and Oceanography: Methods, 10(9): 653-665. https://doi.org/10.4319/lom.2012.10.653 Brand, L. E., Sunda, W. G., Guillard, R. R. L., 1983. Limitation of Marine Phytoplankton Reproductive Rates by Zinc, Manganese, and Iron 1. Limnology and Oceanography, 28(6): 1182-1198. https://doi.org/10.4319/lo.1983.28.6.1182 Bruland, K. W., 1980. Oceanographic Distributions of Cadmium, Zinc, Nickel, and Copper in the North Pacific. Earth and Planetary Science Letters, 47(2): 176-198. https://doi.org/10.1016/0012-821X(80)90035-7 Chen, J., Gaillardet, J., Louvat, P., 2008. Zinc Isotopes in the Seine Rivers, France: A Probe of Anthropogenic Contamination. Environment Science & Technology, 42(17): 6484-6501. https://doi.org/10.1021/es800725z. Chen, J. B., Gaillardet, J., Dessert, C., et al., 2014. Zn Isotope Compositions of the Thermal Spring Waters of La Soufrière Volcano, Guadeloupe Island. Geochimica et Cosmochimica Acta, 127: 67-82. https://doi.org/10.1016/j.gca.2013.11.022 Chen, S., Liu, Y. C., Hu, J. Y., et al., 2015. Zinc Isotopic Compositions of NIST SRM 683 and Whole-Rock Reference Materials. Geostandards and Geoanalytical Research, 40(3): 417-432. https://doi.org/10.1111/j.1751-908X.2015.00377.x Conway, T. M., John, S. G., 2014. The Biogeochemical Cycling of Zinc and Zinc Isotopes in the North Atlantic Ocean. Global Biogeochemical Cycles, 28(10): 1111-1128. https://doi.org/10.1002/2014GB004862 Conway, T. M., John, S. G., 2015. The Cycling of Iron, Zinc and Cadmium in the North East Pacific Ocean— Insights from Stable Isotopes. Geochimica et Cosmochimica Acta, 164: 262-283. https://doi.org/10.1016/j.gca.2015.05.023 Dong, S. F., Wasylenki, L. E., 2016. Zinc Isotope Fractionation during Adsorption to Calcite at High and Low Ionic Strength. Chemical Geology, 447: 70-78. https://doi.org/10.1016/j.chemgeo.2016.10.031 Ellwood, M. J., van den Berg, C. M. G., 2000. Zinc Speciation in the Northeastern Atlantic Ocean. Marine Chemistry, 68(4): 295-306. https://doi.org/10.1016/S0304-4203(99)00085-7 Fernandez, A., Borrok, D. M., 2009. Fractionation of Cu, Fe, and Zn Isotopes during the Oxidative Weathering of Sulfide-Rich Rocks. Chemical Geology, 264(1/2/3/4): 1-12. https://doi.org/10.1016/j.chemgeo.2009.01.024 Fujii, T., Moynier, F., Pons, M. L., et al., 2011. The Origin of Zn Isotope Fractionation in Sulfides. Geochimica et Cosmochimica Acta, 75(23): 7632-7643. https://doi.org/10.1016/j.gca.2011.09.036 Fujii, T., Moynier, F., Telouk, P., et al., 2010. Experimental and Theoretical Investigation of Isotope Fractionation of Zinc between Aqua, Chloro, and Macrocyclic Complexes. The Journal of Physical Chemistry A, 114(7): 2543-2552. https://doi.org/10.1021/jp908642f Gélabert, A., Pokrovsky, O. S., Viers, J., et al., 2006. Interaction between Zinc and Freshwater and Marine Diatom Species: Surface Complexation and Zn Isotope Fractionation. Geochimica et Cosmochimica Acta, 70(4): 839-857. https://doi.org/10.1016/j.gca.2005.10.026 Harrison, G. I., Morel, F. M. M., 1986. Response of the Marine Diatom Thalassiosira Weissflogii to Iron Stress 1. Limnology and Oceanography, 31(5): 989-997. https://doi.org/10.4319/lo.1986.31.5.0989 Hein, J. R., Mizell, K., Koschinsky, A., et al., 2013. Deep-Ocean Mineral Deposits as a Source of Critical Metals for High- and Green-Technology Applications: Comparison with Land-Based Resources. Ore Geology Reviews, 51: 1-14. https://doi.org/10.1016/j.oregeorev.2012.12.001 Hendry, K. R., Andersen, M. B., 2013. The Zinc Isotopic Composition of Siliceous Marine Sponges: Investigating Nature's Sediment Traps. Chemical Geology, 354: 33-41. https://doi.org/10.1016/j.chemgeo.2013.06.025 John, S. G., Geis, R. W., Saito, M. A., et al., 2007. Zinc Isotope Fractionation during High-Affinity and Low-Affinity Zinc Transport by the Marine Diatom Thalassiosira Oceanica. Limnology and Oceanography, 52(6): 2710-2714. https://doi.org/10.4319/lo.2007.52.6.2710 John, S. G., Helgoe, J., Townsend, E., 2018. Biogeochemical Cycling of Zn and Cd and Their Stable Isotopes in the Eastern Tropical South Pacific. Marine Chemistry, 201: 256-262. https://doi.org/10.1016/j.marchem.2017.06.001 John, S. G., Kunzmann, M., Townsend, E. J., et al., 2017. Zinc and Cadmium Stable Isotopes in the Geological Record: A Case Study from the Post-Snowball Earth Nuccaleena Cap Dolostone. Palaeogeography, Palaeoclimatology, Palaeoecology, 466: 202-208. https://doi.org/10.1016/j.palaeo.2016.11.003 John, S. G., Rouxel, O. J., Craddock, P. R., et al., 2008. Zinc Stable Isotopes in Seafloor Hydrothermal Vent Fluids and Chimneys. Earth and Planetary Science Letters, 269(1-2): 17-28. https://doi.org/10.1016/j.epsl.2007.12.011 Köbberich, M., Vance, D., 2017. Kinetic Control on Zn Isotope Signatures Recorded in Marine Diatoms. Geochimica et Cosmochimica Acta, 210: 97-113. https://doi.org/10.1016/j.gca.2017.04.014 Köbberich, M., Vance, D., 2018. Zinc Association with Surface-Bound Iron-Hydroxides on Cultured Marine Diatoms: A Zinc Stable Isotope Perspective. Marine Chemistry, 202: 1-11. https://doi.org/10.1016/j.marchem.2018.01.002 Kunzmann, M., Halverson, G. P., Sossi, P. A., et al., 2013. Zn Isotope Evidence for Immediate Resumption of Primary Productivity after Snowball Earth. Geology, 41(1): 27-30. https://doi.org/10.1130/g33422.1 Liang, L. L., Liu, C. Q., Zhu, X. K., et al., 2020. Zinc Isotope Characteristics in the Biogeochemical Cycle as Revealed by Analysis of Suspended Particulate Matter (SPM) in Aha Lake and Hongfeng Lake, Guizhou, China. Journal of Earth Science, 31(1): 126-140. https://doi.org/10.1007/s12583-017-0957-8 Little, S. H., Sherman, D. M., Vance, D., et al., 2014a. Molecular Controls on Cu and Zn Isotopic Fractionation in Fe-Mn Crusts. Earth and Planetary Science Letters, 396: 213-222. https://doi.org/10.1016/j.epsl.2014.04.021 Little, S. H., Vance, D., Walker-Brown, C., et al., 2014b. The Oceanic Mass Balance of Copper and Zinc Isotopes, Investigated by Analysis of Their Inputs, and Outputs to Ferromanganese Oxide Sediments. Geochimica et Cosmochimica Acta, 125: 673-693. https://doi.org/10.1016/j.gca.2013.07.046 Little, S. H., Vance, D., McManus, J., et al., 2016. Key Role of Continental Margin Sediments in the Oceanic Mass Balance of Zn and Zn Isotopes. Geology, 44(3): 207-210. https://doi.org/10.1130/g37493.1 Liu, S. A., Wu, H. C., Shen, S. Z., et al., 2017. Zinc Isotope Evidence for Intensive Magmatism Immediately before the End-Permian Mass Extinction. Geology, 45(4): 343-346. https://doi.org/10.1130/g38644.1 Liu, S. G., Liu, P. P., Lv, Y., et al., 2019. Cu and Zn Isotope Fractionation during Oceanic Alteration: Implications for Oceanic Cu and Zn Cycles. Geochimica et Cosmochimica Acta, 257: 191-205. https://doi.org/10.1016/j.gca.2019.04.026 Liu, Y. H., Gao, T., Xia, Y. F., et al., 2020. Using Zn Isotopes to Trace Zn Sources and Migration Pathways in Paddy Soils around Mining Area. Environmental Pollution, 267: 115616. https://doi.org/10.1016/j.envpol.2020.115616 Lohan, M. C., Statham, P. J., Crawford, D. W., 2002. Total Dissolved Zinc in the Upper Water Column of the Subarctic North East Pacific. Deep Sea Research Part II: Topical Studies in Oceanography, 49(24/25): 5793-5808. https://doi.org/10.1016/S0967-0645(02)00215-1 Lv, Y., Liu, S. G., Wu, H. C., et al., 2018. Zn-Sr Isotope Records of the Ediacaran Doushantuo Formation in South China: Diagenesis Assessment and Implications. Geochimica et Cosmochimica Acta, 239: 330-345. https://doi.org/10.1016/j.gca.2018.08.003 Maréchal, C. N., Nicolas, E., Douchet, C., et al., 2000. Abundance of Zinc Isotopes as a Marine Biogeochemical Tracer. Geochemistry, Geophysics, Geosystems, 1(5): 1015. https://doi.org/10.1029/1999GC000029 Martin, J. H., Gordon, R. M., Fitzwater, S., et al., 1989. Vertex: Phytoplankton/Iron Studies in the Gulf of Alaska. Deep Sea Research Part A Oceanographic Research Papers, 36(5): 649-680. https://doi.org/10.1016/ 0198-0149(89)90144-1 doi: 10.1016/0198-0149(89)90144-1 Mavromatis, V., González, A. G., Dietzel, M., et al., 2019. Zinc Isotope Fractionation during the Inorganic Precipitation of Calcite-Towards a New pH Proxy. Geochimica et Cosmochimica Acta, 244: 99-112. https://doi.org/10.1016/j.gca.2018.09.005 Mccoy-West, A. J., Fitton, J. G., Pons, M. L., et al., 2018. The Fe and Zn Isotope Composition of Deep Mantle Source Regions: Insights from Baffin Island Picrites. Geochimica et Cosmochimica Acta, 238: 542-562. https://doi.org/10.1016/j.gca.2018.07.021 Morel, F. M. M., Milligan, A.J., Saito, M. A., 2014. Marine Bioinorganic Chemistry: The Role of Trace Metals in the Oceanic Cycles of Major Nutrients. Treatise on Geochemistry (Second Edition), 8: 123-150. https://doi.org/10.1016/B0-08-043751-6/06108-9. Morel, F. M. M., Reinfelder, J. R., Roberts, S. B., et al., 1994. Zinc and Carbon Co-Limitation of Marine Phytoplankton. Nature, 369(6483): 740-742. https://doi.org/10.1038/369740a0 Moynier, F., Vance, D., Fujii, T., et al., 2017. The Isotope Geochemistry of Zinc and Copper. Reviews in Mineralogy and Geochemistry, 82(1): 543-600. https://doi.org/10.2138/rmg.2017.82.13 Pichat, S., Douchet, C., Albarède, F., 2003. Zinc Isotope Variations in Deep-Sea Carbonates from the Eastern Equatorial Pacific over the Last 175 ka. Earth and Planetary Science Letters, 210(1/2): 167-178. https://doi.org/10.1016/S0012-821X(03)00106-7 Pokrovsky, O. S., Pokrovski, G. S., Gélabert, A., et al., 2005. Speciation of Zn Associated with Diatoms Using X-Ray Absorption Spectroscopy. Environmental Science & Technology, 39(12): 4490-4498. https://doi.org/10.1021/es0480419 Reiswig, H. M., 1971. Particle Feeding in Natural Populations of Three Marine Demosponges. The Biological Bulletin, 141(3): 568-591. https://doi.org/10.2307/1540270 Samanta, M., Ellwood, M. J., Sinoir, M., et al., 2017. Dissolved Zinc Isotope Cycling in the Tasman Sea, SW Pacific Ocean. Marine Chemistry, 192: 1-12. https://doi.org/10.1016/j.marchem.2017.03.004 Shaked, Y., Xu, Y., Leblanc, K., et al., 2006. Zinc Availability and Alkaline Phosphatase Activity in Emiliania Huxleyi: Implications for Zn-P Co-Limitation in the Ocean. Limnology and Oceanography, 51(1): 299-309. https://doi.org/10.4319/lo.2006.51.1.0299 Shields, W. R., Murphy, T. J., Garner, E. L., 1964. Absolute Isotopic Abundance Ratio and the Atomic Weight of a Reference Sample of Copper. Journal of Research of the National Bureau of Standards Section A: Physics and Chemistry, 68A(6): 589-592. https://doi.org/10.6028/jres.068a.056 Sinoir, M., Butler, E. C. V., Bowie, A. R., et al., 2012. Zinc Marine Biogeochemistry in Seawater: A Review. Marine and Freshwater Research, 63(7): 644-657. https://doi.org/10.1071/mf11286 Sossi, P. A., Nebel, O., O'Neill, H. S. C., et al., 2018. Zinc Isotope Composition of the Earth and Its Behaviour during Planetary Accretion. Chemical Geology, 477: 73-84. https://doi.org/10.1016/j.chemgeo.2017.12.006 Sunda, W. G., Huntsman, S. A., 1992. Feedback Interactions between Zinc and Phytoplankton in Seawater. Limnology and Oceanography, 37(1): 25-40. https://doi.org/10.4319/lo.1992.37.1.0025 Sweere, T. C., Dickson, A. J., Jenkyns, H. C., et al., 2018. Isotopic Evidence for Changes in the Zinc Cycle during Oceanic Anoxic Event 2 (Late Cretaceous). Geology, 46(5): 463-466. https://doi.org/10.1130/g40226.1 Sweere, T. C., Dickson, A. J., Jenkyns, H. C., et al., 2020. Zinc- and Cadmium-Isotope Evidence for Redox-Driven Perturbations to Global Micronutrient Cycles during Oceanic Anoxic Event 2 (Late Cretaceous). Earth and Planetary Science Letters, 546: 116427. https://doi.org/10.1016/j.epsl.2020.116427 Twining, B. S., Baines, S. B., 2013. The Trace Metal Composition of Marine Phytoplankton. Annual Review of Marine Science, 5(1): 191-215. https://doi.org/10.1146/annurev-marine-121211-172322 United States National Research Council, 2000. Zinc, in Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press, Washington, D.C., 442-501. Vance, D., de Souza, G. F., Zhao, Y., et al., 2019. The Relationship between Zinc, Its Isotopes, and the Major Nutrients in the North-East Pacific. Earth and Planetary Science Letters, 525: 115748. https://doi.org/10.1016/j.epsl.2019.115748 Vance, D., Little, S. H., Archer, C., et al., 2016. The Oceanic Budgets of Nickel and Zinc Isotopes: The Importance of Sulfidic Environments as Illustrated by the Black Sea. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 374(2081): 20150294. https://doi.org/10.1098/rsta.2015.0294 Vance, D., Little, S. H., de Souza, G. F., et al., 2017. Silicon and Zinc Biogeochemical Cycles Coupled through the Southern Ocean. Nature Geoscience, 10(3): 202-206. https://doi.org/10.1038/ngeo2890 Wang, X., Liu, S. G., Wang, Z. R., et al., 2018. Zinc and Strontium Isotope Evidence for Climate Cooling and Constraints on the Frasnian-Famennian (~372 Ma) Mass Extinction. Palaeogeography, Palaeoclimatology, Palaeoecology, 498: 68-82. https://doi.org/10.1016/j.palaeo.2018.03.002 Wang, Y., Zhu, X.K., 2010. Application of Zn Isotopes to Study of Mineral Deposits: A Review. Mineral Deposits, 29(5): 843-852 (in Chinese with English abstract). http://www.researchgate.net/publication/309698136_Application_of_Zn_isotopes_to_study_of_mineral_deposits_A_review_in_Chinese Wang, Z.X., Liu, S. A., Li, M.L., et al., 2020. Advances on Application of Zinc Isotope as a Tracer for Deep Carbon Cycles. Earth Science, 45(6): 1967-1976 (in Chinese with English abstract). https://doi.org/10.3799/dqkx.2020.159 Wang, Z. Z., Liu, S. G., Liu, J. G., et al., 2017. Zinc Isotope Fractionation during Mantle Melting and Constraints on the Zn Isotope Composition of Earth's Upper Mantle. Geochimica et Cosmochimica Acta, 198: 151-167. https://doi.org/10.1016/j.gca.2016.11.014 Yan, B., Zhu, X. K., He, X. X., et al., 2019. Zn Isotopic Evolution in Early Ediacaran Ocean: A Global Signature. Precambrian Research, 320: 472-483. https://doi.org/10.1016/j.precamres.2018.11.021 Yang, Y. H., Zhang, X. C., Liu, S. A., et al., 2018. Calibrating NIST SRM 683 as a New International Reference Standard for Zn Isotopes. Journal of Analytical Atomic Spectrometry, 33(10): 1777-1783. https://doi.org/10.1039/c8ja00249e Zimmermann, T., Mohamed, A. F., Reese, A., et al., 2020. Zinc Isotopic Variation of Water and Surface Sediments from the German Elbe River. Science of the Total Environment, 707: 135219. https://doi.org/10.1016/j.scitotenv.2019.135219 Zhao, M. Y., Tarhan, L. G., Zhang, Y. Y., et al., 2021. Evaluation of Shallow-Water Carbonates as a Seawater Zinc Isotope Archive. Earth and Planetary Science Letters, 553: 116599. https://doi.org/10.1016/j.epsl.2020.116599 Zhao, Y., Vance, D., Abouchami, W., et al., 2014. Biogeochemical Cycling of Zinc and Its Isotopes in the Southern Ocean. Geochimica et Cosmochimica Acta, 125: 653-672. https://doi.org/10.1016/j.gca.2013.07.045 王跃, 朱祥坤, 2010. 锌同位素在矿床学中的应用: 认识与进展. 矿床地质, 29(5): 843-852. doi: 10.3969/j.issn.0258-7106.2010.05.007 王照雪, 刘盛遨, 李孟伦, 等, 2020. 深部碳循环的锌同位素示踪研究进展. 地球科学, 45(6): 1967-1976. doi: 10.3799/dqkx.2020.159 期刊类型引用(3)
1. 唐文波,彭佑多,何术东. 多点位保压取样器结构设计与强度分析. 矿业工程研究. 2024(03): 1-10 .  百度学术
百度学术2. 李梓靖,焦港辉,苟航. 铁和锌同位素在表生系统研究中的应用. 皮革制作与环保科技. 2023(05): 181-182 .  百度学术
百度学术3. Yiwen Lü,Sheng-Ao Liu. Cu and Zn Isotopic Evidence for the Magnitude of Organic Burial in the Mesoproterozoic Ocean. Journal of Earth Science. 2022(01): 92-99 .  必应学术
必应学术其他类型引用(3)
-









 下载:
下载:

 下载:
下载:
 百度学术
百度学术



