Niche Specificity and Potential Functions of Microbial Communities in Karst Caves as Exampled by Panlong Cave in Guilin City, Guangxi
-
摘要: 开展岩溶洞穴不同生境微生物与环境因子间的研究,对阐明深部生物圈微生物的多样性、潜在功能及环境驱动机制具有重要意义.以广西桂林盘龙洞为例,通过对细菌16S rRNA高通量测序系统研究了洞穴7种小生境细菌群落的空间分布特征及其与环境因子之间的关系.研究发现温度是驱动盘龙洞细菌群落组成的重要因素,微生物群落组成及潜在生态功能均具有生境特异性,不同生境具有独有的细菌指示类群.滴水、干燥石笋表面生物膜、湿润石笋表面生物膜3个生境与洞穴氮循环密切相关,风化结皮、沉积物、岩壁3个生境与洞穴微生物固定二氧化碳关系紧密.此外,洞穴细菌通过密切的代谢交换形成协作的正相关关系,暗示着微生物在洞穴这一极端环境中的生存策略.Abstract: Karst caves serve as the natural laboratories to study subsurface biosphere, which provide ideal places to investigate microbial diversity and their interactions between microbes and environments. Guilin is one of the typical areas with developed karst landscape. Deep investigation of cave microbes in different niches and their response to environmental variables will provide valuable information on subsurface biosphere in terms of microbial diversity, function and interaction with environments. To this end, Samples were collected from overlying soils, sediments, cave wall, weathered crusted on cave walls, microbial biofilm on stalagmite surface and dripping water in the Panlong Cave in Guiling City to study microbial communities and their correlation with environmental variables via high throughput sequencing of 16S rRNA. Results show that bacterial communities show high specificity to niches and were dominated by Actinomycete and Proteobacteria. Each niche has its own specific indicator group. For example, Paemibacillus was the indicator for microbial biofilm on the dry surface of stalagmite, whilst Acidobacteriaceae and Pseudomonas were the indicator groups in microbial biofilm on the wet surface of stalagmite. Pseudomonadales and Branhamella were the indicator groups in dripping water, whereas indicator groups in overlying soils included Mycobacterium and Nocardioides. Bacillus and Gp-7 were indicators in sediments, whilst Chromatiales, Soilrubrobacteraceae and Rubrobcter were indicators in cave wall and Methylobacterium in weathered crust. Temperature was demonstrated to be the main variable impacting bacterial communities in the Panlong Cave as indicated by redundancy analysis. Tax4fun2 analysis shows that microbes participated in carbon and nitrogen cycles also varied with niches. Microbes in dripping water, microbial biofilm on dry/wet surface of stalagmite were closely related to nitrogen cycle. On the contrast, those in weathered crust, sediments and cave wall were more involved in carbon fixation. Co-occurrence network analysis inferred that corporation was the main strategy for microbial groups to survive in the oligotrophic karst caves.
-
Key words:
- karst cave /
- subsurface biosphere /
- carbon fixation /
- nitrogen cycle /
- co-occurrence network /
- geomicrobiology
-
图 2 桂林盘龙洞微生物群落结构及不同生境中微生物群落的指示类群
a.相对丰度前10的门的组成;b.相对丰度前15的属组成;c.基于LEfSe分析确定的不同生境的指示类群(LDA SCORE > 4);d.基于ASVs水平的微生物群落非度量多维尺度分析(NMDS);DW.滴水;S.沉积物;WC.风化结皮;W.岩壁;DS.干石笋表面生物膜;OS.上覆土壤;WS.湿石笋表面生物膜
Fig. 2. Microbial community compositions and microbial indicator groups of different niches in the Panlong Cave, Guilin City
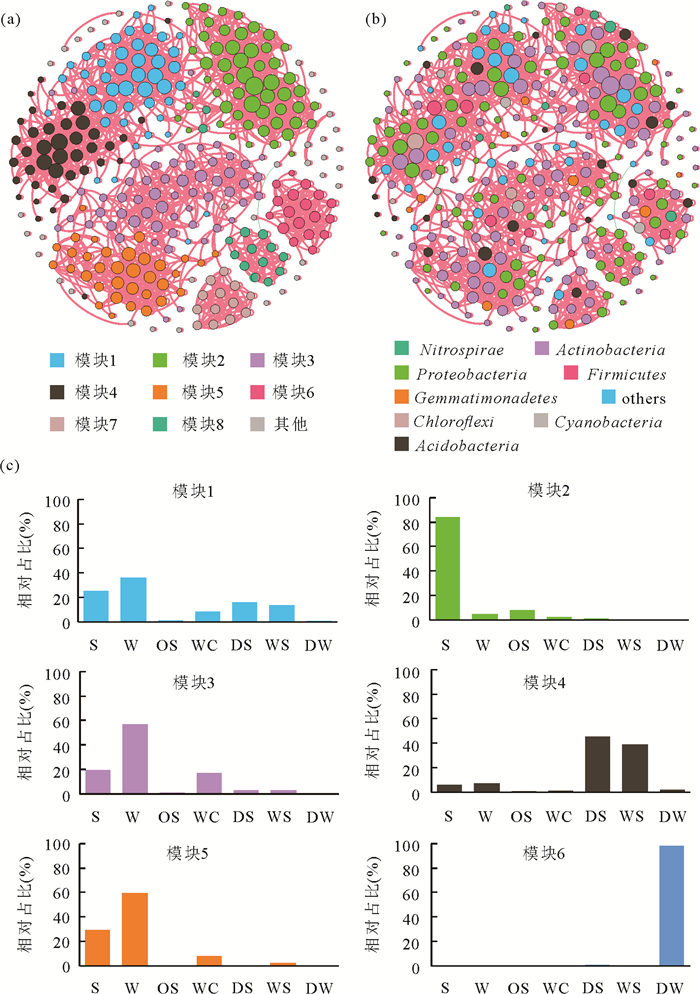
图 5 盘龙洞细菌群落的共现网络分析.(a)按模块着色;(b)按门类着色;(c)来自不同生境的节点在网络模块中所占比例
每个节点的大小与其连接数量成正比,其中粉色代表正相关,绿色代表负相关.S、W、OS、WC、DW、WS、DS分别代表沉积物,岩壁,上覆土壤,风化结皮,滴水,湿石笋表面生物膜,干石笋表面生物膜
Fig. 5. The co-occurrence network analysis of bacterial communities colored by taxonomy (a) and by modules (b) in the Panlong Cave, Guilin City, Guangxi. The node size is proportional to the number of connections. Positive links were in pink and negative ones in green. (c) Relative abundance of bacterial ASVs within individual modules in the networks
表 1 广西桂林盘龙洞洞穴中各个生境样本α多样性指数
Table 1. α diversity index of microbial communities in various habitats in the Panlong Cave, Guilin City, Guangxi
生境 Simpson Chao1 ACE Shannon S 0.98±0.02a 1 449.64±218.07bc 1 454.32±218.59bc 8.13±0.67ab W 0.98±0.03a 1 372.14±397.49bc 1 374.13±402.37bcd 8.12±0.91ab OS 0.98±0.03a 2 244.26±562.91a 2 224.21±553.77a 8.95±1.12a WC 0.98±0.01a 1 053.27±377.47cd 1 058.01±373.47cd 7.67±0.95b DW 0.86±0.01b 965.02±361.88d 982.32±377.49d 5.6±0.45c WS 0.98±0.03a 1 517.85±145.02b 1 519.34±139.96b 8.29±0.79ab DS 0.99±0.00a 1 667.17±331.51b 1 662.63±335.29b 8.97±0.27a 注:不同字母(a~d)表示生境中存在显著性差异(P<0.05);S.沉积物,W.岩壁,OS.上覆土壤,WC.风化结皮,DW.滴水,WS.湿石笋表面生物膜,DS.干石笋表面生物膜. 表 2 广西桂林盘龙洞内各样品的理化参数
Table 2. Physicochemical parameters of samples in the Panlong Cave in Guilin City, Guangxi
样品名 Cl-
(mg/kg)SO42-
(mg/kg)K+
(mg/kg)Na+
(mg/kg)Si4+
(mg/kg)Ca/Mg pH TOC(%) C/N 温度(℃) PLD1W 2.04±0.13 108.72±12.57 8.64±0.11 7.14±1.85 15.36±0.50 8.21±0.05 9.14±0.03 2.68 82.76 10.80 PLD1S 1.32±0.08 35.70±0.42 1.98±0.04 8.10±9.62 5.94±7.51 7.47±0.10 8.44±0.04 1.82 11.49 10.80 PLD2W 2.16±0.11 296.82±2.03 52.50±1.88 6.84±0.44 37.92±5.57 4.08±2.12 8.39±0.03 0.73 5.64 13.60 PLD2S 0.66±0.14 20.34±0.27 0.78±0.06 6.36±0.70 16.56±2.13 18.9±0.70 8.39±0.01 1.45 7.66 13.60 PLD3W 3.36±0.62 30.66±9.12 6.54±0.13 12.72±7.02 51.78±5.63 5.06±0.09 9.04±0.01 1.57 122.95 17.10 PLD3S 10.98±0.11 134.52±1.97 22.44±0.91 6.96±0.71 14.52±1.24 2.10±0.02 8.41±0.04 0.77 7.21 17.10 PLD4W 2.34±0.14 61.92±0.49 4.44±0.07 7.74±0.92 17.58±1.86 8.10±0.06 8.95±0.02 2.79 246.82 16.40 PLD4S 24.66±0.94 606.84±20.39 42.06±0.95 8.34±9.34 6.06±7.34 4.50±0.03 7.80±0.01 0.58 6.00 16.40 PLD5W 6.24±0.08 97.68±9.17 5.58±0.47 8.46±0.46 17.88±2.39 22.13±0.48 8.41±0.04 2.93 50.65 17.40 PLD5S 23.64±0.17 1 682.04±77.99 148.62±3.90 8.28±0.77 19.32±1.70 8.18±0.01 7.83±0.04 0.38 5.32 17.40 PLD6W 2.04±0.07 40.68±0.23 5.34±0.06 9.72±2.06 27.84±8.95 30.63±0.57 8.73±0.02 3.67 63.71 17.90 PLD6S 10.32±1.21 1 374.60±18.00 36.00±1.30 6.48±0.42 14.64±3.97 14.29±0.13 8.15±0.03 0.54 5.37 17.90 PLD7W 2.82±0.09 26.04±0.31 6.06±6.07 8.10±2.05 21.60±15.73 3.54±0.35 9.19±0.03 5.48 302.34 18.30 PLD7S 8.70±0.04 63.84±1.52 12.72±0.37 10.02±1.93 33.66±14.77 6.41±0.14 8.62±0.02 4.46 172.81 18.30 PLD8W 3.24±0.00 18.18±0.90 29.52±23.06 10.68±6.12 19.20±7.74 3.63±0.71 9.05±0.07 1.81 201.72 18.60 PLD8S 1.62±0.07 14.46±0.34 222.54±88.59 15.48±4.98 28.50±4.40 0.94±0.45 8.46±0.03 3.71 146.12 18.60 PLD9W 3.36±0.00 21.54±0.37 1.38±0.04 10.68±4.85 21.42±6.90 31.29±12.42 8.73±0.01 2.77 147.06 18.70 PLD9S 6.18±0.07 68.10±0.20 72.84±83.33 15.06±4.36 28.98±5.75 17.81±10.48 8.14±0.02 5.76 156.64 18.70 PLD10W 5.22±0.01 68.82±0.20 0.84±0.29 8.52±1.25 22.68±2.85 26.42±1.83 9.26±0.04 4.08 353.98 19.30 PLD10S 0.54±0.02 34.98±24.30 32.52±0.85 9.42±1.74 22.50±8.22 8.07±0.2 8.57±0.03 0.28 4.02 19.30 -
Barton, H. A., Giarrizzo, J. G., Suarez, P., et al., 2014. Microbial Diversity in a Venezuelan Orthoquartzite Cave is Dominated by the Chloroflexi (Class Ktedonobacterales) and Thaumarchaeota Group Ⅰ. 1c. Frontiers in Microbiology, 5: 615. https://doi.org/10.3389/fmicb.2014.00615 Bastida, F., Selevsek, N., Torres, I. F., et al., 2015. Soil Restoration with Organic Amendments: Linking Cellular Functionality and Ecosystem Processes. Scientific Reports, 5: 15550. https://doi.org/10.1038/srep15550 Bokulich, N. A., Thorngate, J. H., Richardson, P. M., et al., 2014. Microbial Biogeography of Wine Grapes is Conditioned by Cultivar, Vintage, and Climate. Proceedings of the National Academy of Sciences of the United States of America, 111(1): E139-E148. https://doi.org/10.1073/pnas.1317377110 Bradford, M. A., McCulley, R. L., Crowther, T. W., et al., 2019. Cross-Biome Patterns in Soil Microbial Respiration Predictable from Evolutionary Theory on Thermal Adaptation. Nature Ecology & Evolution, 3(2): 223-231. https://doi.org/10.1038/s31559-018-0771-4 Cai, Y. F., Zhou, X., Shi, L. M., et al., 2020. Atmospheric Methane Oxidizers are Dominated by Upland Soil Cluster Alpha in 20 Forest Soils of China. Microbial Ecology, 80(4): 859-871. https://doi.org/10.1007/s00248-020-01570-1 Caporaso, J. G., Kuczynski, J., Stombaugh, J., et al., 2010. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nature Methods, 7(5): 335-336. https://doi.org/10.1038/nmeth.f.303 Cheeptham, N., Sadoway, T., Rule, D., et al., 2013. Cure from the Cave: Volcanic Cave Actinomycetes and Their Potential in Drug Discovery. International Journal of Speleology, 42(1): 35-47. https://doi.org/10.5038/1827-806x.42.1.5 Cheng, S. B., Liu, A. S., Cui, S., et al., 2021. Mineralization Process of Permian Karst Bauxite in Western Guangxi. Earth Science, 46(8): 2697-2710(in Chinese with English abstract). Cheng, X. Y., Liu, X. Y., Wang, H. M., et al., 2021a. USCγ Dominated Community Composition and Cooccurrence Network of Methanotrophs and Bacteria in Subterranean Karst Caves. Microbiology Spectrum, 9(1): e0082021. https://doi.org/10.1128/spectrum.00820-21 Cheng, X. Y., Yun, Y., Wang, H. M., et al., 2021b. Contrasting Bacterial Communities and Their Assembly Processes in Karst Soils under Different Land Use. Science of the Total Environment, 751: 142263. https://doi.org/10.1016/j.scitotenv.2020.142263 Claesson, M., O'Sullivan, Ó., Wang, Q., et al., 2009. Comparative Analysis of Pyrosequencing and a Phylogenetic Microarray for Exploring Microbial Community Structures in the Human Distal Intestine. PloS One, 4(8): e6669. https://doi.org/10.1371/journal.pone.0006669 Davis, M. C., Messina, M. A., Nicolosi, G., et al., 2020. Surface Runoff Alters Cave Microbial Community Structure and Function. PLoS One, 15(5): e0232742. https://doi.org/10.1371/journal.pone.0232742 Debora, R., Tugba, O., 2016. Carbon Dioxide Sequestration through Microbially-Induced Calcium Carbonate Precipitation Using Ureolytic Aquatic Microorganisms. Abstracts of Papers of the American Chemical Society, 251: 672-680. Edgar, R. C., 2010. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics, 26(19): 2460-2461. https://doi.org/10.1093/bioinformatics/btq461 Edgar, R. C., Haas, B. J., Clemente, J. C., et al., 2011. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics, 27(16): 2194-2200. https://doi.org/10.1093/bioinformatics/btr381 Fang, B. Z., Salam, N., Han, M. X., et al., 2017. Insights on the Effects of Heat Pretreatment, pH, and Calcium Salts on Isolation of Rare Actinobacteria from Karstic Caves. Frontiers in Microbiology, 8: 1535. https://doi.org/10.3389/fmicb.2017.01535 Gulecal-Pektas, Y., 2016. Bacterial Diversity and Composition in Oylat Cave (Turkey) with Combined Sanger/Pyrosequencing Approach. Polish Journal of Microbiology, 65: 69-75. https://doi.org/10.5604/17331331.1197277 Han, Q., Ma, Q., Chen, Y., et al., 2020. Variation in Rhizosphere Microbial Communities and Its Association with the Symbiotic Efficiency of Rhizobia in Soybean. The ISME Journal, 14(8): 1915-1928. https://doi.org/10.1038/s31396-020-0648-9 He, Z. L., Gentry, T. J., Schadt, C. W., et al., 2007. GeoChip: A Comprehensive Microarray for Investigating Biogeochemical, Ecological and Environmental Processes. The ISME Journal, 1(1): 67-77. https://doi.org/10.1038/ismej.2007.2 Herbst, F. A., Jehmlich, N., von Bergen, M., et al., 2018. Sulfur-34S and 36S Stable Isotope Labeling of Amino Acids for Quantification (SULAQ34/36) of Proteome Analyses. Microbial Proteomics. Humana Press, New York, 163-174. https://doi.org/10.1007/978-1-4939-8695-8_12 Ivanova, A. A., Zhelezova, A. D., Chernov, T. I., et al., 2020. Linking Ecology and Systematics of Acidobacteria: Distinct Habitat Preferences of the Acidobacteriia and Blastocatellia in Tundra Soils. PLos One, 15(3): e0230157. https://doi.org/10.1371/journal.pone.0230157 Jones, D. S., Lyon, E., Macalady, J., 2008. Geomicrobiology of Biovermiculations from the Frasassi Cave System, Italy. Journal of Cave and Karst Studies, 70: 78-93. Knief, C., 2015. Diversity and Habitat Preferences of Cultivated and Uncultivated Aerobic Methanotrophic Bacteria Evaluated Based on PMOA as Molecular Marker. Frontiers in Microbiology, 6: 1346. https://doi.org/10.3389/fmicb.2015.01346 Knief, C., Lipski, A., Dunfield, P. F., 2003. Diversity and Activity of Methanotrophic Bacteria in Different Upland Soils. Applied and Environmental Microbiology, 69(11): 6703-6714. https://doi.org/10.1128/aem.69.11.6703-6714.2003 Kontro, M., Lignell, U., Hirvonen, M. R., et al., 2005. pH Effects on 10 Streptomyces spp. Growth and Sporulation Depend on Nutrients. Letters in Applied Microbiology, 41(1): 32-38. https://doi.org/10.1111/j.1472-765x.2005.01727.x Kraft, B., Tegetmeyer, H. E., Sharma, R., et al., 2014. The Environmental Controls That Govern the End Product of Bacterial Nitrate Respiration. Science, 345(6197): 676-679. https://doi.org/10.1126/science.1254070 Kranjc, A., 2011. The Origin and Evolution of the Term "Karst". Procedia—Social and Behavioral Sciences, 19: 567-570. https://doi.org/10.1016/j.sbspro.2011.05.170 Kuypers, M. M. M., Marchant, H. K., Kartal, B., et al., 2018. The Microbial Nitrogen-Cycling Network. Nature Reviews Microbiology, 16(5): 263-276. https://doi.org/10.1038/nrmicro.2018.9 Lavoie, K. H., Winter, A. S., Read, K. J. H., et al., 2017. Comparison of Bacterial Communities from Lava Cave Microbial Mats to Overlying Surface Soils from Lava Beds National Monument, USA. PLoS One, 12(2): e0169339. https://doi.org/10.1371/journal.pone.0169339 Lewin, G. R., Carlos, C., Chevrette, M. G., et al., 2016. Evolution and Ecology of Actinobacteria and Their Bioenergy Applications. Annual Review of Microbiology, 70: 235-254. https://doi.org/10.1146/annurev-micro-102215-095748 Lian, B., Xiao, L. L., Sun, Q. B., 2017. Ecological Effects of the Microbial Weathering of Silicate Minerals. Acta Geologica Sinica (English Edition), 91(Suppl. 1): 150-152. https://doi.org/10.1111/1755-6724.13231 Ma, L. Y., Huang, X. P., Wang, H. M., et al., 2021. Microbial Interactions Drive Distinct Taxonomic and Potential Metabolic Responses to Habitats in Karst Cave Ecosystem. Microbiology Spectrum, 9(2): e0115221. https://doi.org/10.1128/Spectrum.01152-21 Melillo, J. M., Frey, S. D., DeAngelis, K. M., et al., 2017. Long-Term Pattern and Magnitude of Soil Carbon Feedback to the Climate System in a Warming World. Science, 358(6359): 101-105. doi: 10.1126/science.aan2874 Morris, B. E. L., Henneberger, R., Huber, H., et al., 2013. Microbial Syntrophy: Interaction for the Common Good. FEMS Microbiology Reviews, 37(3): 384-406. https://doi.org/10.1111/1574-6976.12019 Mozafari, M., Sajjadian, M., Sorninia, Y., et al., 2020. Hydrogeology and Geomorphology of Bisetun Aquifer (NW Iran): Interesting Example of Deep Endokarst. Carbonates and Evaporites, 35(4): 1-19. https://doi.org/10.1007/s13146-020-00636-y Nelson, M. B., Martiny, A. C., Martiny, J. B. H., 2016. Global Biogeography of Microbial Nitrogen-Cycling Traits in Soil. Proceedings of the National Academy of Sciences of the United States of America, 113(29): 8033-8040. https://doi.org/10.1073/pnas.1601070113 Ortiz, M., Legatzki, A., Neilson, J. W., et al., 2014. Making a Living While Starving in the Dark: Metagenomic Insights into the Energy Dynamics of a Carbonate Cave. The ISME Journal, 8(2): 478-491. https://doi.org/10.1038/ismej.2013.159 Poisot, T., Gravel, D., 2014. When is an Ecological Network Complex? Connectance Drives Degree Distribution and Emerging Network Properties. Peer Journal, 2: e251. https://doi.org/10.7717/peerj.251 Porter, M. L., Engel, A. S., Kane, T. C., et al., 2009. Productivity-Diversity Relationships from Chemolithoautotrophically Based Sulfidic Karst Systems. International Journal of Speleology, 38: 27-40. https://doi.org/10.5038/1827-806x.38.1.4 Pratscher, J., Vollmers, J., Wiegand, S., et al., 2018. Unravelling the Identity, Metabolic Potential and Global Biogeography of the Atmospheric Methane-Oxidizing Upland Soil Cluster α. Environmental Microbiology, 20(3): 1016-1029. https://doi.org/10.1111/1462-2920.14036 Proctor, L. M., 1997. Nitrogen-Fixing, Photosynthetic, Anaerobic Bacteria Associated with Pelagic Copepods. Aquatic Microbial Ecology, 12: 105-113. https://doi.org/10.3354/ame012105 Pu, G. Z., Lü, Y. N., Dong, L. N., et al., 2019. Profiling the Bacterial Diversity in a Typical Karst Tiankeng of China. Biomolecules, 9(5): 187. https://doi.org/10.3390/biom9050187 Puissant, J., Jones, B., Goodall, T., et al., 2019. The pH Optimum of Soil Exoenzymes Adapt to Long Term Changes in Soil pH. Soil Biology and Biochemistry, 138: 107601. https://doi.org/10.1016/j.soilbio.2019.107601 Radita, R., Suwanto, A., Kurosawa, N., et al., 2018. Firmicutes is the Predominant Bacteria in Tempeh. International Food Research Journal, 25(6): 2313-2320. Reitschuler, C., Spötl, C., Hofmann, K., et al., 2016. Archaeal Distribution in Moonmilk Deposits from Alpine Caves and Their Ecophysiological Potential. Microbial Ecology, 71(3): 686-699. https://doi.org/10.1007/s00248-015-0727-z Riquelme, C., Marshall Hathaway, J. J., de L N Enes Dapkevicius, M., et al., 2015. Actinobacterial Diversity in Volcanic Caves and Associated Geomicrobiological Interactions. Frontiers in Microbiology, 6: 1342. https://doi.org/10.3389/fmicb.2015.01342 Sauro, F., Mecchia, M., Tringham, M., et al., 2020. Speleogenesis of the World's Longest Cave in Hybrid Arenites (Krem Puri, Meghalaya, India). Geomorphology, 359: 107160. https://doi.org/10.1016/j.geomorph.2020.107160 Smit, E., Leeflang, P., Gommans, S., et al., 2001. Diversity and Seasonal Fluctuations of the Dominant Members of the Bacterial Soil Community in a Wheat Field as Determined by Cultivation and Molecular Methods. Applied and Environmental Microbiology, 67(5): 2284-2291. https://doi.org/10.1128/aem.67.5.2284-2291.2001 Sun, X. L., Xu, Z. H., Xie, J. Y., et al., 2022. Bacillus velezensis Stimulates Resident Rhizosphere Pseudomonas stutzeri for Plant Health through Metabolic Interactions. The ISME Journal, 16(3): 774-787. https://doi.org/10.1038/s31396-021-01125-3 Tang, K., Baskaran, V., Nemati, M., 2009. Bacteria of the Sulphur Cycle: An Overview of Microbiology, Biokinetics and Their Role in Petroleum and Mining Industries. Biochemical Engineering Journal, 44(1): 73-94. https://doi.org/10.1016/j.bej.2008.12.011 Tetu, S. G., Breakwell, K., Elbourne, L. D. H., et al., 2013. Life in the Dark: Metagenomic Evidence that a Microbial Slime Community is Driven by Inorganic Nitrogen Metabolism. The ISME Journal, 7(6): 1227-1236. https://doi.org/10.1038/ismej.2013.14 Veress, M. J., 2020. Karst Types and Their Karstification. Journal of Earth Science, 31(3): 621-634. https://doi.org/10.1007/s12583-020-1306-x Wang, Q., Zhang, Z. H., Du, R., et al., 2019. Richness of Plant Communities Plays a Larger Role Than Climate in Determining Responses of Species Richness to Climate Change. Journal of Ecology, 107: 1944-1955. https://doi.org/10.1111/1365-2745.13148 Wang, W. F., Ma, Y. T., Ma, X., et al., 2012. Diversity and Seasonal Dynamics of Airborne Bacteria in the Mogao Grottoes, Dunhuang, China. Aerobiologia, 28(1): 27-38. https://doi.org/10.1007/s10453-011-9208-0 Wang, X. Y., He, T. H., Gen, S. Y., et al., 2020. Soil Properties and Agricultural Practices Shape Microbial Communities in Flooded and Rainfed Croplands. Applied Soil Ecology, 147: 103449. https://doi.org/10.1016/j.apsoil.2019.103449 Yang, S. H., Ahn, H., Kim, B. S., et al., 2017. Comparison of Bacterial Communities in Leachate from Decomposing Bovine Carcasses. Asian-Australasian Journal of Animal Sciences, 30(11): 1660-1666. https://doi.org/10.5713/ ajas.17.0553 doi: 10.5713/ajas.17.0553 Yang, Y., Li, T., Wang, Y. Q., et al., 2021. Linkage between Soil Ectoenzyme Stoichiometry Ratios and Microbial Diversity Following the Conversion of Cropland into Grassland. Agriculture, Ecosystems & Environment, 314: 107418. https://doi.org/10.1016/j.agee.2021.107418 Yun, Y. A., Wang, H. M., Man, B. Y., et al., 2016. The Relationship between pH and Bacterial Communities in a Single Karst Ecosystem and Its Implication for Soil Acidification. Frontiers in Microbiology, 7: 1955. https://doi.org/10.3389/fmicb.2016.01955 Yun, Y. A., Wang, W. Q., Wang, H. M., et al., 2018. Seasonal Variation of Bacterial Community and Their Functional Diversity in Drip Water from a Karst Cave. Chinese Science Bulletin, 63(36): 3932-3944. https://doi.org/10.1360/n972018-00627 Yun, Y. A., Xiang, X., Wang, H. M., et al., 2016. Five-Year Monitoring of Bacterial Communities in Dripping Water from the Heshang Cave in Central China: Implication for Paleoclimate Reconstruction and Ecological Functions. Geomicrobiology Journal, 33(7): 1-11. https://doi.org/10.1080/01490451.2015.1062062 Zelezniak, A., Andrejev, S., Ponomarova, O., et al., 2015. Metabolic Dependencies Drive Species Co-Occurrence in Diverse Microbial Communities. Proceedings of the National Academy of Sciences of the United States of America, 112(20): 6449-6454. https://doi.org/10.1073/pnas.1421834112 Zeng, L. L., Tian, J. Q., Chen, H., et al., 2019. Changes in Methane Oxidation Ability and Methanotrophic Community Composition across Different Climatic Zones. Journal of Soils and Sediments, 19(2): 533-543. https://doi.org/10.1007/s11368-018-2069-1 Zhang, Y., Yang, Q. S., Ling, J., et al., 2021. The Diversity of Alkane-Degrading Bacterial Communities in Seagrass Ecosystem of the South China Sea. Ecotoxicology, 30: 1799-1807. https://doi.org/10.1007/s10646-021-02450-1 Zhao, R., Wang, H. M., Cheng, X. Y., et al., 2018. Upland Soil Cluster γ Dominates the Methanotroph Communities in the Karst Heshang Cave. FEMS Microbiology Ecology, 94(12): fiy192. https://doi.org/10.1093/femsec/fiy192 Zhu, H. Z., Zhang, Z. F., Zhou, N., et al., 2019. Diversity, Distribution and Co-Occurrence Patterns of Bacterial Communities in a Karst Cave System. Frontiers in Microbiology, 10: 1726. https://doi.org/10.3389/fmicb.2019.01726 Zhu, H. Z., Zhang, Z. F., Zhou, N., et al., 2021. Bacteria and Metabolic Potential in Karst Caves Revealed by Intensive Bacterial Cultivation and Genome Assembly. Applied and Environmental Microbiology, 87(6): e02440-e02420. https://doi.org/10.1128/aem.02440-20 程顺波, 刘阿睢, 崔森, 等, 2021. 桂西二叠纪喀斯特型铝土矿地质成矿过程. 地球科学, 46(8): 2697-2710. doi: 10.3799/dqkx.2020.295 -









 下载:
下载: