Interaction between Stibnite and Microbial Communities Enriched from Tailings at Xikuangshan Coupling with Bacterial Community Succession
-
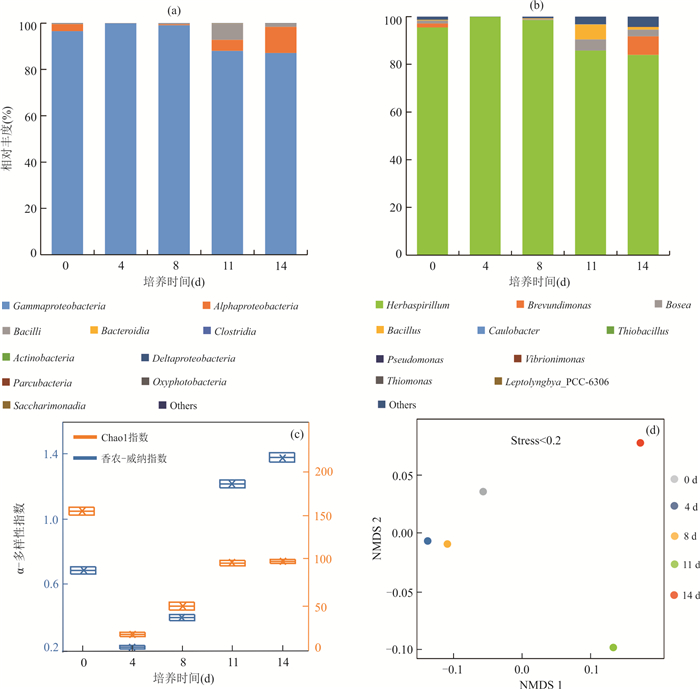
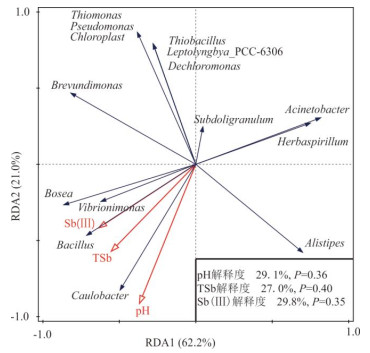
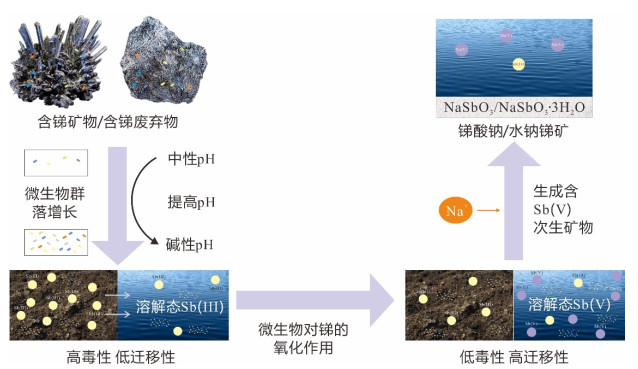
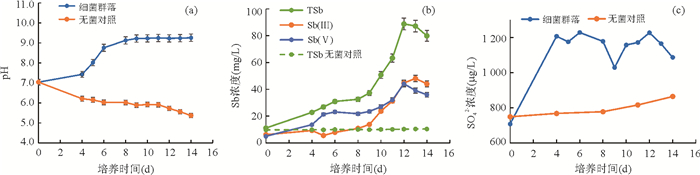
摘要: 为了从群落水平上探讨细菌对辉锑矿的溶解释放作用,从湖南锡矿山尾矿库尾矿渣中富集了细菌群落,开展了其与辉锑矿相互作用的实验研究.通过湿化学方法检测实验过程中pH和锑含量的变化、用16S rRNA高通量测序检测实验过程中细菌群落的变化,用X射线粉晶衍射仪(XRD)检测微生物与矿物相互作用后矿物相的变化,进而揭示群落水平上微生物与辉锑矿相互作用的机制及相互作用过程中细菌群落的演替.结果表明在好氧条件下细菌群落可以以乳酸钠为碳源进行异养生长,造成溶液pH的升高,进而导致辉锑矿的溶解.实验初期辉锑矿溶解释放的三价锑迅速被氧化成五价锑,但第9 d之后三价锑开始在溶液中积累,在实验结束时浓度比五价锑略高.溶液中五价锑的浓度在第12 d之前持续上升,之后下降,直至实验结束.溶液中锑含量的升高,对细菌群落进行了强烈的筛选作用.实验过程中细菌群落均以对As/Sb等多种重金属具有抗性的草螺菌属(Herbaspirillum)占绝对优势,但其相对丰度在第11 d之后有所下降.具有锑氧化能力的细菌类群如副球菌属(Paracoccus)、博斯氏菌属(Bosea)等在群落中的相对丰度较低,导致辉锑矿溶解释放出来的三价锑不能被完全氧化.XRD结果显示辉锑矿与细菌群落作用后生成了含有五价锑的锑酸钠,这与溶液中五价锑浓度的下降相吻合.该结果为进一步理解锡矿山自然环境中细菌群落对含锑矿物的溶解、锑的氧化、迁移和转化的影响提供了新的认识.Abstract: To better understand microbial dissolution and oxidation of stibnite at the community level, microbial communities were enriched from the tailings in the Xikuangshan tailings pond, Hunan Province, which were further used to study microbial interaction with stibnite. pH, total Sb, Sb(Ⅴ), Sb(Ⅲ) and SO42- were measured periodically. 16S rRNA high-throughput sequencing and X-ray powder diffractometer (XRD) were exploited to detect compositions of microbial communities and mineral phases, respectively. The results show that microbial communities can grow with sodium lactate and increase the pH of the solution under aerobic condition, which facilitated the dissolution of stibnite. The concentration of Sb(Ⅴ) accumulated in the solution due to the oxidation of the released Sb(Ⅲ), with an decrease after the 12th day. The Sb(Ⅲ) concentration in the solution increased after the 9th day and was a little bit higher than that of Sb(Ⅴ) at the end of the experiments. The incomplete oxidation of Sb(Ⅲ) may result from the low relative abundances of antimony-oxidizing bacteria such as Paracoccus and Bosea in the enriched culture. The variation of solution chemistry, particularly the Sb concentration, exerted strong selection on microbial communities. Microbial communities were dominated by Herbaspirillum sp., with strong resistance to metals, such as and Sb. However their relative abundances decreased after 11 days' interaction potentially due to the accumulation of Sb in the system. Secondary Sb(Ⅴ)-bearing mineral, sodium antimonite, was detected at the end of the experiments in the biotic systems, which matched well with the decrease of the concentration of Sb(Ⅴ) in the solution. Our results further confirm the mechanism of microbial dissolution of stibnite followed by oxidation and transformation of minerals at the community level, which provides new insights of understanding the impact of microbial communities on the dissolution of Sb-bearing secondary minerals, oxidation, migration and formation of antimony in natural environments.
-
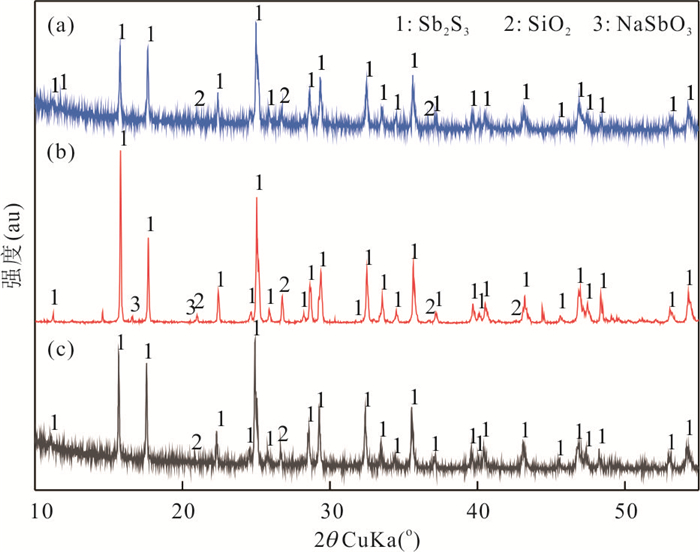
表 1 微生物菌群与辉锑矿相互作用前后固体样品中矿物组成的XRD半定量结果
Table 1. Semi-quantification of mineral composition in the solution before and after the interaction with microbial communities
Sb2S3 SiO2 NaSbO3 原始辉锑矿粉末 97.2% 2.8% - 实验组 83.8% 15.1% 1.1% 对照组 96.1% 3.9% - 表 2 Bosea sp. AS-1、Paracoccus sp. XT0.6和菌群与辉锑矿相互作用有关参数的对比
Table 2. Parameter comparison of the interactions between stibnite with Bosea sp. AS-1, Paracoccus sp. XT0.6 and microbial communities
菌株名称或菌群 体系结束时的pH 是否产生次生矿物 次生矿物名称及分子式 锑的释放率(%) 释放后锑的氧化率(%) XT0.6 9.3 是 水钠锑矿(NaSb(OH)6) 12.5 100 AS-1 6.8 否 / 5.8 100 富集菌群 9.1 是 锑酸钠(NaSbO3) 10.7 50.1 -
Ali, A., Li, M., Su, J., et al., 2022. Brevundimonas diminuta Isolated from Mines Polluted Soil Immobilized Cadmium (Cd2+) and Zinc (Zn2+) through Calcium Carbonate Precipitation: Microscopic and Spectroscopic Investigations. Science of Total Environment, 813: 152668. https://doi.org/10.1016/j.scitotenv.2021.152668 Biver, M., Shotyk, W., 2012. Stibnite (Sb2S3) Oxidative Dissolution Kinetics from pH 1 to 11. Geochimica et Cosmochimica Acta, 79: 127-139. https://doi.org/10.1016/j.gca.2011.11.033 Caporaso, J. G., Kuczynski, J., Stombaugh, J., et al., 2010. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Gut Microbes, 7(5): 335-336. https://doi.org/10.1038/nmeth.f.303 Edgar, R. C., 2013. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Frontiers in Plant Science, 10(10): 996-998. https://doi.org/10.1038/nmeth.2604 Edgar, R. C., Haas, B. J., Clemente, J. C., et al., 2011. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics, 27(16): 2194-2200. https://doi.org/10.1093/bioinformatics/btr381 Fang, Q., Huang, T., Wang, N., et al., 2021. Effects of Herbaspirillum sp. P5-19 Assisted with Alien Soil Improvement on the Phytoremediation of Copper Tailings by Vetiveria zizanioides L. Environmental Science and Pollution Research, 28(45): 64757-64768. Fu, Z., Wu, F., Mo, C., et al., 2016. Comparison of Arsenic and Antimony Biogeochemical Behavior in Water, Soil and Tailings from Xikuangshan, China. Science of the Total Environment, 539: 97-104. https://doi.org/10.1016/j.scitotenv.2015.08.146 Govarthanan, M., Lee, G. W., Park, J. H., et al., 2014. Bioleaching Characteristics, Influencing Factors of Cu Solubilization and Survival of Herbaspirillum sp. GW103 in Cu Contaminated Mine Soil. Chemosphere, 109: 42-48. https://doi.org/10.1016/j.chemosphere.2014.02.054 Hamamura, N., Fukushima, K., Itai, T, 2013. Identification of Antimony- and Arsenic-Oxidizing Bacteria Associated with Antimony Mine Tailing. Microbes and Environments, 28(2): 257-263. https://doi.org/10.1264/jsme2.me12217 Hamamura, N., Nakajima, N., Yamamura, S., 2020. Draft Genome Sequence of the Antimony-Oxidizing Pseudomonas sp. Strain SbOxS1, Isolated from Stibnite Mine Tailing Soil. Microbiology Resource Announcements, 9(49): e01218-20. https://doi.org/10.1128/MRA.01218-20 He, M., Wang, X., Wu, F., et al., 2012. Antimony Pollution in China. Science of Total Environment, 421/422: 41-50. https://doi.org/10.1016/j.scitotenv.2011.06.009 Jiang, N., Li, X. Q., Zhou, A. G., et al., 2020. Effect of pH Value and Fe(Ⅲ) on the Oxidative Dissolution of Stibnite. Bulletin of Geological Science and Technology, 39(4): 76-84 (in Chinese with English abstract). Li, J., Wang, Q., Li, M., et al., 2015. Proteomics and Genetics for Identification of a Bacterial Antimonite Oxidase in Agrobacterium tumefaciens. Environmental Science & Technology, 49(10): 5980-5989. https://doi.org/10.1021/es506318b Li, J., Wang, Q., Zhang, S. Z., et al., 2013. Phylogenetic and Genome Analyses of Antimony-Oxidizing Bacteria Isolated from Antimony Mined Soil. International Biodeterioration & Biodegradation, 76: 76-80. https://doi.org/10.1016/j.ibiod.2012.06.009 Li, Y. B., Lin, H. Z., Gao, P., et al., 2022. Synergistic Impacts of Arsenic and Antimony Co-Contamination on Diazotrophic Communities. Microbial Ecology, 84(1): 44-58. https://doi.org/10.1007/s00248-021-01824-6 Lialikova, N. N., 1974. Stibiobacter senarmontii: A New Microorganism Oxidizing Antimony. Iranian Journal of Public Health, 43(6): 941-948. Lu, X., Zhang, Y., Liu, C., et al., 2018. Characterization of the Antimonite- and Arsenite-Oxidizing Bacterium Bosea sp. AS-1 and Its Potential Application in Arsenic Removal. Journal of Hazardous Materials, 359: 527-534. https://doi.org/10.1016/j.jhazmat.2018.07.112 Magoč, T., Salzberg, S. L, 2011. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics, 27(21): 2957-2963. https://doi.org/10.1093/bioinformatics/btr507 Multani, R. S., Feldmann, T., Demopoulos, G. P., 2016. Antimony in the Metallurgical Industry: A Review of Its Chemistry and Environmental Stabilization Options. Hydrometallurgy, 164: 141-153. https://doi.org/10.1016/j.hydromet.2016.06.014 Praburaman, L., Park, S. H., Cho, M., et al., 2017. Significance of Diazotrophic Plant Growth-Promoting Herbaspirillum sp. GW103 on Phytoextraction of Pb and Zn by Zea Mays L. Environmental Science and Pollution Research, 24(3): 3172-3180. Quast, C., Pruesse, E., Yilmaz, P., et al., 2012. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Research, 41(D1): D590-D596. https://doi.org/10.1093/nar/gks1219 Rathi, M., Yogalakshmi, K. N., 2021. Brevundimonas diminuta MYS6 Associated Helianthus annuus L. for Enhanced Copper Phytoremediation. Chemosphere, 263: 128195. https://doi.org/10.1016/j.chemosphere.2020.128195 Rhine, E. D., Phelps, C. D., Young, L. Y., 2006. Anaerobic Arsenite Oxidation by Novel Denitrifying Isolates. Environmental Microbiology, 8(5): 899-908. https://doi.org/10.1111/j.1462-2920.2005.00977.x Sforza, E., Pastore, M., Sanchez, S. S., et al., 2018. Bioaugmentation as a Strategy to Enhance Nutrient Removal: Symbiosis between Chlorella protothecoides and Brevundimonas diminuta. Bioresource Technology Reports, 4: 153-158. https://doi.org/10.1016/j.biteb.2018.10.007 Shi, Z., Cao, Z., Qin, D., et al., 2013. Correlation Models between Environmental Factors and Bacterial Resistance to Antimony and Copper. PLoS One, 8(10): e78533. https://doi.org/10.1371/journal.pone.0078533 Singh, N., Marwa, N., Mishra, S. K., et al., 2016. Brevundimonas Diminuta Mediated Alleviation of Arsenic Toxicity and Plant Growth Promotion in Oryza Sativa L. Ecotoxicology and Environmental Safety, 125: 25-34. https://doi.org/10.1016/j.ecoenv.2015.11.020 Singh, S., Kumar, V., Gupta, P., et al., 2021. The Synergy of Mercury Biosorption through Brevundimonas sp. IITISM22: Kinetics, Isotherm, and Thermodynamic Modeling. Journal of Hazardous Materials, 415: 125653. https://doi.org/10.1016/j.jhazmat.2021.125653 Skeaff, J. M., Beaudoin, R., Wang, R., et al., 2013. Transformation/Dissolution Examination of Antimony and Antimony Compounds with Speciation of the Transformation/Dissolution Solutions. Integrated Environmental Assessment and Management, 9(1): 98-113. https://doi.org/10.1002/ieam.1339 Sun, X. X., Kong, T. L., Häggblom, M. M., et al., 2020. Chemolithoautotropic Diazotrophy Dominates the Nitrogen Fixation Process in Mine Tailings. Environmental Science & Technology, 54(10): 6082-6093. https://doi.org/10.1021/acs.est.9b07835 Sun, X. X., Li, B. Q., Han, F., et al., 2019. Impacts of Arsenic and Antimony Co-Contamination on Sedimentary Microbial Communities in Rivers with Different Pollution Gradients. Microbial Ecology, 78(3): 589-602. https://doi.org/10.1007/s00248-019-01327-5 Terry, L. R., Kulp, T. R., Wiatrowski, H., et al., 2015. Microbiological Oxidation of Antimony (Ⅲ) with Oxygen or Nitrate by Bacteria Isolated from Contaminated Mine Sediments. Applied and Environmental Microbiology, 81(24): 8478-8488. https://doi.org/10.1128/AEM.01970-15 Torma, A. E., Gabra, G. G, 1977. Oxidation of Stibnite by Thiobacillus Ferrooxidans. Antonie van Leeuwenhoek, 43(1): 1-6. https://doi.org/10.1007/BF02316204 Wang, N. N., Zhang, S. H., He, M. C, 2018. Bacterial Community Profile of Contaminated Soils in a Typical Antimony Mining Site. Environmental Science and Pollution Research, 25(1): 141-152. https://doi.org/10.1007/s11356-016-8159-y Wang, Q., Garrity, G. M., Tiedje, J. M., et al., 2007. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Mucosal Immunology, 73(16): 5261-5267. https://doi.org/10.1128/aem.00062-07 Xiang, L., Liu, C., Liu, D., et al., 2022. Antimony Transformation and Mobilization from Stibnite by an Antimonite Oxidizing Bacterium Bosea sp. AS-1. Journal of Environmental Sciences (China), 111: 273-281. https://doi.org/10.1016/j.jes.2021.03.042 Xiao, E. Z., Krumins, V., Dong, Y. R., et al., 2016. Microbial Diversity and Community Structure in an Antimony-Rich Tailings Dump. Applied Microbiology and Biotechnology, 100(17): 7751-7763. https://doi.org/10.1007/s00253-016-7598-1 Zhang, G. P., Ouyang, X. X., Li, H. X., et al., 2016. Bioremoval of Antimony from Contaminated Waters by a Mixed Batch Culture of Sulfate-Reducing Bacteria. International Biodeterioration and Biodegradation, 115: 148-155. https://doi.org/10.1016/j.ibiod.2016.08.007 江南, 李小倩, 周爱国, 等, 2020. pH值和氧化剂对硫化锑氧化溶解的影响机制. 地质科技通报, 39(4): 76-84. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ202004010.htm -









 下载:
下载: