CO2 Solubility in Shanxi Formation Water of Ordos Basin
-
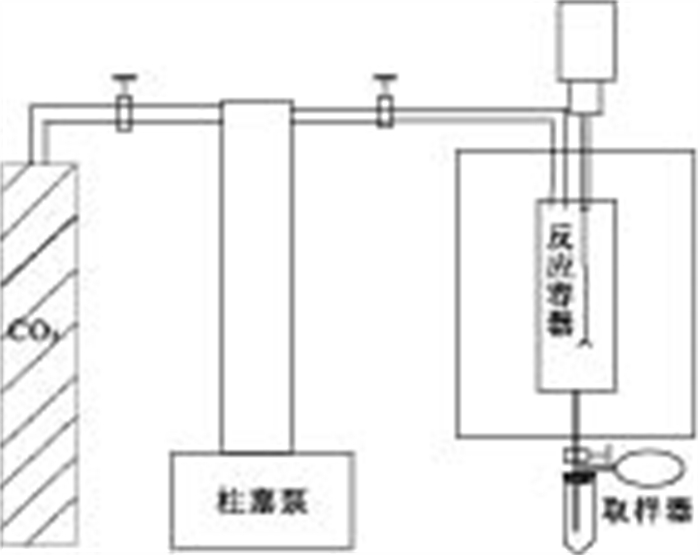
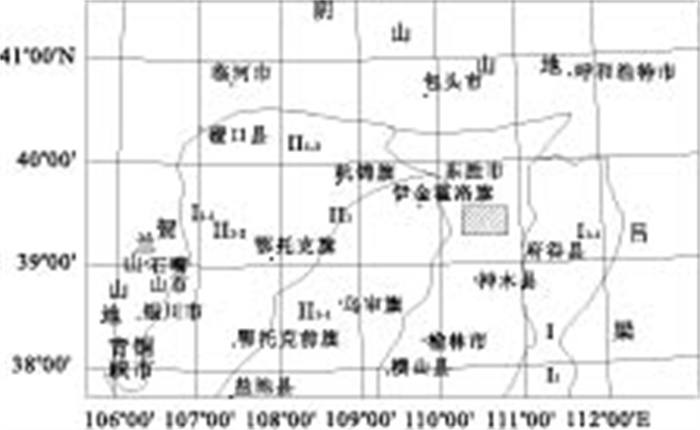
摘要: 实施CO2的地质储存是目前公认的减缓全球变暖的有效途径之一.潜在的储存场所包括衰竭的油气藏、深部不可开采煤层及深部咸水层.其中, 深部咸水层储存潜力最大.在发挥作用的诸多机理中, 溶解埋存具有埋存量大、作用时间较长以及安全性高的特点.在评价深部咸水含水层CO2溶解储存潜力时, 溶解度是一个关键参数.提出了测定咸水含水层地层水CO2溶解度的方法, 并将其实际应用于鄂尔多斯盆地山西组地层水.鄂尔多斯盆地是我国重要的能源基地, CO2排放量大, 排放浓度高.采集了野外实地水样, 进行了化学成分分析, 并人工合成该水样; 测定了40~80 ℃、8~12 MPa条件下CO2在该水样中的溶解度, 其结果可为评价鄂尔多斯盆地深部咸水含水层埋存能力提供依据.Abstract: Geological storage is one of the most effective means to reduce the anthropogenic greenhouse gas emissions to mitigate the worsening global warming. Depleted oil-gas reservoirs, coal seams and deep saline aquifers are potential sites for CO2 geological storage of which saline aquifer has the greatest potential for sequestration. Among the many effective mechanisms, dissolving storage is characterized by large storage capacity, long action time and high safety. When evaluating the storage capacity of a deep saline aquifer, CO2 solubility becomes a key parameter. In this paper, an experimental method is proposed and used to measure the CO2 solubility in Shanxi Formation water. Ordos Basin is an important energy base for China which releases a lot of high concentration CO2. Studies show CO2 geological storage is possible in Ordos Basin since its Shanxi Formation forms many source-reservoir-cap assemblages, and it is of great importance both in theory and practice to probe into CO2 solubility in Shanxi Formation water of Ordos Basin. In this paper, chemical composition of Shanxi Formation water collected from the Ordos Basin were analyzed. CO2 solubilities in the artificial synthetic Shanxi Formation waterwere measured at 40-80℃, 8-12 MPa pressure. The results can be used for the evaluation of the CO2 storage capacity in deep saline aquifer of Ordos Basin.
-
Key words:
- geological storage /
- Ordos basin /
- solubility /
- groundwater /
- hydrogeology
-
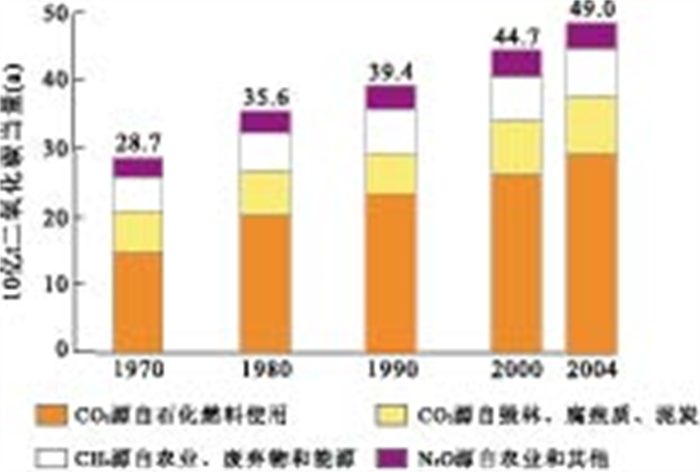
表 1 鄂尔多斯盆地山西组地下水化学成分
Table 1. Chemical composition of Shanxi Formation groundwater in Ordos basin
项目 ρB(mg·L-1) CB(mmol·L-1) pH 6.90 K+ 67.80 1.734 Na+ 2405.00 104.565 Ca2+ 888.50 22.168 Mg2+ 32.79 1.349 NH4+ <0.01 <0.001 Al3+ <0.02 — Cl- 5151.49 145.317 SO42- 29.10 0.303 HCO3- 642.01 10.521 表 2 人工合成鄂尔多斯盆地山西组地下水实际用量
Table 2. Recipe for synthetic Shanxi Formation groundwater in Ordos basin
试剂 级别 质量(g) NaCl 分析纯 5.5016 KCl 分析纯 0.1267 CaCl2 分析纯 2.4598 MgCl2 分析纯 0.1283 NaHCO3 分析纯 0.8838 表 3 人工合成山西组水样CO2溶解度数据
Table 3. Solubility of CO2 in synthetic Shanxi Formation water
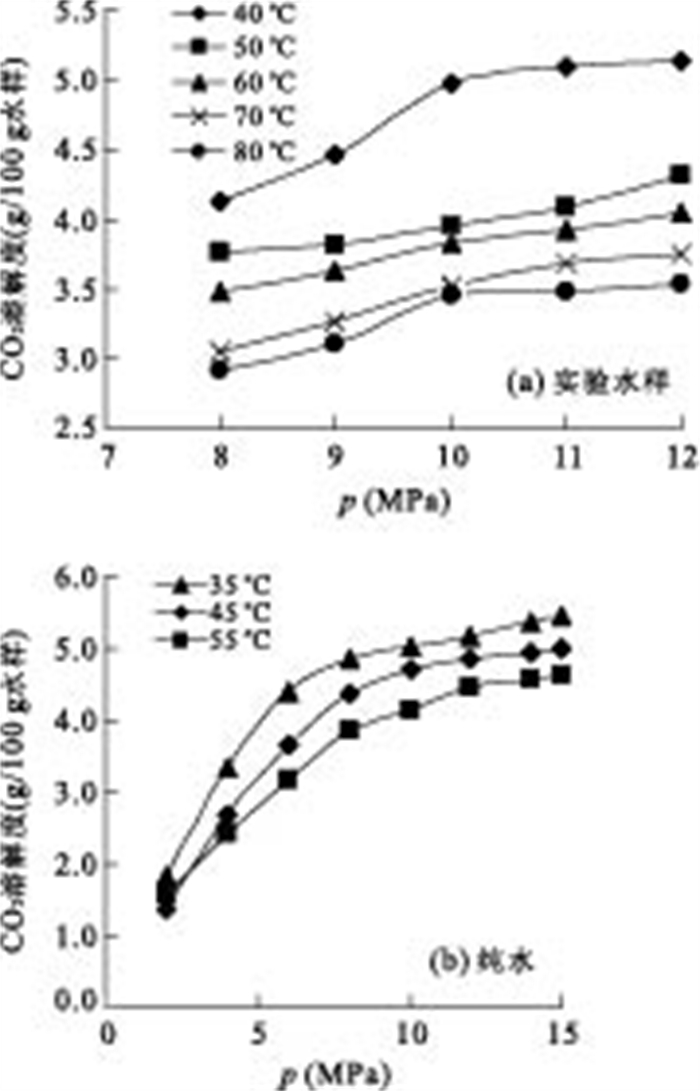
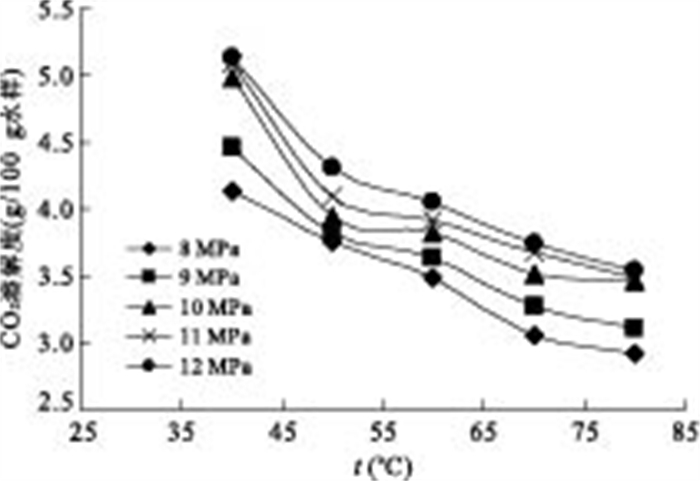
p(MPa) t(℃) CO2溶解度(g/100g水样) 8 40 4.1325 8 50 3.7660 8 60 3.4672 8 70 2.8024 8 80 2.9234 9 40 4.4582 9 50 3.8240 9 60 3.6137 9 70 3.2732 9 80 3.1099 10 40 4.9088 10 50 3.8848 10 60 3.8328 10 70 3.6542 10 80 3.4597 11 40 5.1454 11 50 4.0946 11 60 3.9191 11 70 3.6859 11 80 3.3427 12 40 5.0948 12 50 4.3054 12 60 4.0520 12 70 3.6379 12 80 3.4382 -
Darwish, N.A., Hilal, N., 2010. A simple model for the prediction of CO2 solubility in H2O-NaCl system at geological sequestration conditions. Desalination, 260(1-3): 114-118. doi: 10.1016/j.desal.2010.04.056 Deparment of Chemistry, East China University of Science and Technology, et al., 2003. Analytical chemistry. Higher Education Press, Beijing, 425 (in Chinese). Duan, Z.H., Sun, R., 2003. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2 000 bar. Chem. Geol. , 193(3-4): 257-271. doi: 10.1016/S0009-2541(02)00263-2 Ellis, A.J., Golding, R.M., 1963. The solubility of carbon dioxide above 100 ℃ in water and in sodium chloride solutions. American Journal of Science, 261(1): 47-60. doi: 10.2475/ajs.261.1.47 Ferrentinoa, G., Barlettaa, D., Balaban, M.O., et al., 2010. Measurement and prediction of CO2 solubility in sodium phosphate monobasic solutions for food treatment with high pressure carbon dioxide. The Journal of Supercritical Fluids, 52(1): 142-150. doi: 10.1016/j.supflu.2009.10.005 Hou, G.C., Zhang, M.S., 2008. The survey and research on groundwater in Ordos basin. Geological Publishing House, Beijing, 85 (in Chinese). Li, X.C., Liu, Y.F., Bai, B., et al., 2006. Ranking and screening of CO2 saline aquifer storage zones in China. Chinese Journal of Rock Mechanics and Engineering, 25(5): 963-968 (in Chinese with English abstract). http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&cmd=prlinks&retmode=ref&id=21866545 Li, X.C., Ohsumia, T., Koide, H., et al., 2005. Near-future perspective of CO2 aquifer storage in Japan: site selection and capacity. Energy, 30(11-12): 2360-2369. doi: 10.1016/j.energy.2004.08.026 Liu, Y.H., Hou, M.Q., Yang, G.Y., et al., 2011. Solubility of CO2 in aqueous solutions of NaCl, KCl, CaCl2 and their mixed salts at different temperatures and pressures. The Journal of Supercritical Fluids, 56(2): 125-129. doi: 10.1016/j.supflu.2010.12.003 Ren, X.K., Cui, Y.J., Bu, X.P., et al., 2010. Analysis on CO2 storage potentiality in Ordos basin. Energy of China, 32(1): 29-32 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZGLN201001015.htm Shen, P.P., Liao, X.W., 2009. The technology of carbon dioxide stored in geological media and enhanced oil recovery. Petroleum Industry Press, Beijing, 39 (in Chinese). Teng, H., Yamasaki, A., 1998. Solubility of liquid CO2 in synthetic sea water at temperatures from 278 K to 293 K and pressures from 6.44 MPa to 29.49 MPa, and densities of the corresponding aquous solutions. J. Chem. Eng. Data. , 43(1): 2-5. doi: 10.1021/je9700737 Wiebe, R., Gaddy, V.L., 1939. The solubility in water of carbon dioxide at 50 ℃, 75 ℃ and 100 ℃, at pressures to 700 atmospheres. J. Am. Chem. Soc. , 61(2): 315-318. doi: 10.1021/ja01871a025 Wiebe, R., Gaddy, V.L., 1940. The solubility of carbon dioxide in water at various temperatures from 12 to 40 ℃ and at pressures to 500 atmospheres. J. Am. Chem. Soc. , 62(4): 815-817. doi: 10.1021/ja01861a033 Portier, S., Rochelle, C., 2005. Modeling CO2 solubility in pure water and NaCl-type waters from 0 to 300 ℃ and from 1 to 300 bar: application to the Utsira Formation at Sleipner. Chemical Geology, 217(3-4): 187-199. doi: 10.1016/j.chemgeo.2004.12.007 Zeng, R.S., Sun, S., Chen, D.Z., et al., 2004. Decrease carbon dioxide emission into the atmosphere-underground disposal of carbon dioxide. Bulletin of National Natural Science Foundation of China, 4: 196-200 (in Chinese with English abstract). http://d.wanfangdata.com.cn/periodical/zgkxjj200404002 华东理工大学化学系等, 2003. 分析化学. 北京: 高等教育出版社, 425. 侯光才, 张茂省, 2008. 鄂尔多斯盆地地下水勘查系统. 北京: 地质出版社, 85. 李小春, 刘延锋, 白冰, 等, 2006. 中国深部咸水含水层CO2储存优先区域选择. 岩石力学与工程学报, 25(5): 963-968. doi: 10.3321/j.issn:1000-6915.2006.05.015 任相坤, 崔永君, 步学朋, 等, 2010. 鄂尔多斯盆地CO2地质封存潜力分析. 中国能源, 32(1): 29-32. doi: 10.3969/j.issn.1003-2355.2010.01.006 沈平平, 廖新维, 2009. 二氧化碳地质埋存与提高石油采收率技术. 北京: 石油工业出版社, 39. 曾荣树, 孙枢, 陈代钊, 等, 2004. 减少二氧化碳向大气层的排放——二氧化碳地下储存研究. 中国科学基金, 4: 196-200. doi: 10.3969/j.issn.1000-8217.2004.04.002 -
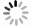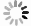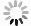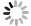








 下载:
下载: