The Analytical Development of Low-Temperature Particulate Fe Speciation
-
摘要: 元素铁因其在地球早期环境演化和在海洋初级生产力中的重要角色, 是目前全球变化与地球化学研究的热点.铁元素的相态分析, 对于深入了解其在环境中迁移、转化及生物的吸收利用, 都具有重要意义.回顾沉积物中铁的化学相态分析方法发展过程, 从早期的简单相态分析(degree of pyritization, DOP), 元素的顺序提取法(Tesser方法), 到Raiswell和Poulton等学者提出针对Fe元素特定的相态分析法等.重点介绍较为常用的三步提取法和其在表生地球化学研究中的应用; 结合目前国际上铁地球化学循环研究进展, 提出Fe的化学相态分析的改进建议.Abstract: Iron geochemistry has rapidly attracted great interest for global change research over the last two decades due to its significant role in the evolution of earth environment and in marine primary productivity. The speciation of particulate iron is critical for better understanding of iron transformation, transitivity and bioavailability. This paper systematically reviews the analytical development of low-temperature particulate Fe speciation, including the degree of pyritization (DOP), Tessier's sequential extraction method, and specific iron extraction procedure. The application of three-step iron extraction is introduced, and some suggestions are given for the future iron speciation analysis based on the latest development of iron geochemical study.
-
Key words:
- iron /
- sediments /
- chemical speciation /
- development /
- geochemistry
-
图 1 胶体态、纳米颗粒态等的粒径范围(改绘自Raiswell and Canfield, 2012)
Fig. 1. Size ranges of particles, colloids, nanoparticles and aqueous species in relation to filterable iron
图 2 铁的生物地球化学循环中海洋储库的输入和输出示意图(改绘自Raiswell, 2006)
Fig. 2. Inputs and outputs to marine reservoirs in the iron biogeochemical cycle
表 1 颗粒态Fe的三步萃取法(Poulton and Raiswell, 2002)
Table 1. The three-step extraction of particulate iron
分类 提取试剂 Fe的主要赋存形态 FeHR 连二亚硫酸钠缓冲溶液pH=4.8反应1 h 无定形以及晶体态的含Fe氧化物(除磁铁矿) FePR 12 mol/L HCl煮沸2 min 磁铁矿和部分层状硅酸盐矿物中含有的Fe,例如绿泥石、绿脱石、海绿石以及黑云母等 FeU HF-HClO4-HNO3 与硅酸盐结合的Fe 表 2 颗粒态Fe的七步提取法(Poulton and Canfield, 2005)
Table 2. The seven-step extraction of particulate iron
步骤 分类 主要赋存矿物 所用试剂 1 可交换态 吸附的含铁矿物 1 mol/L MgCl2 pH=7.0,反应2 h 2 碳酸盐结合态的铁(Fecarb) 菱铁矿,铁白云石 1 mol/L CH3COONa pH=4.5(用CH3COOH调),50 ℃反应48 h 3 易被提取的氧化物态(Feox1) 水铁矿,纤铁矿 1 mol/L NH2OH·HCl加入25%(v/v)HOAc 4 能被提取的氧化物态(Feox2) 针铁矿,赤铁矿和正方针铁矿 0.35 mol/L HAc和0.2 mol/L柠檬酸钠缓冲50 g/L的连二亚硫酸钠溶液(pH=4.8)反应2 h 5 磁铁矿(Femag) 磁铁矿 0.2 mol/L草酸铵和0.17 mol/L草酸溶液,pH=3.2,反应6 h 6 低活性层状硅酸盐矿物(FePRS) 绿脱石、绿泥石、海绿石、黑云母等 12 mol/L HCl(5 ml)煮沸,1 min 7 不活性的含铁硅酸盐(FeU) 残渣(450 ℃ 8 h)用6 mol/L HCl反应24 h 表 3 铁各化学相态提取主要方法比较
Table 3. The comparison between the main method of particulate iron extraction
提出时间 提取方法 所用主要试剂 提取物 文献来源 1970年 单步提取法 12 mol/L浓HCl沸煮 反应性Fe Berner, 1970 1979年 五步提取法 MgCl2、醋酸钠、盐酸羟胺溶液、硝酸双氧水溶液以及HF-HClO4混合溶液 金属元素各化学相态 Tessier et al., 1979 1989年 三步提取法 草酸、连二亚硫酸钠溶液、HF-H2SO4混合溶液 铁的各个化学相态 Canfield, 1989 1994年 三步提取法 连二亚硫酸钠溶液、冷盐酸、热盐酸 铁的各个化学相态 Raiswell et al., 1994 2005年 七步提取法 MgCl2、醋酸钠、盐酸羟胺溶液、连二亚硫酸钠溶液、草酸铵溶液、12 mol/L浓HCl以及6 mol/L的HCl 铁的各个化学相态 Poulton and Canfield, 2005 -
Anderson, T.F., Raiswell, R., 2004. Sources and Mechanisms for the Enrichment of Highly Reactive Iron in Euxinic Black Sea Sediments. American Journal of Science, 304(3): 203-233. doi: 10.2475/ajs.304.3.203 Berner, R.A., 1970. Sedimentary Pyrite Formation. American Journal of Science, 268(1): 1-23. doi: 10.2475/ajs.268.1.1 Boyd, P.W., Watson, A.J., Law, C.S., et al., 2000. A Mesoscale Phytoplankton Bloom in the Polar Southern Ocean Stimulated by Iron Fertilization. Nature, 407: 695-702. doi: 10.1038/35037500 Calmano, W., Forstner, U., 1983. Chemical Extraction of Heavy Metals in Polluted River Sediments in Central Europe. Science of the Total Environment, 28(1-3): 77-90. doi: 10.1016/S0048-9697(83)80009-6 Canfield, D.E., 1989. Reactive Iron in Marine Sediments. Geochimica et Cosmochimica Acta, 53: 619-632. doi: 10.1016/0016-7037(89)90005-7 Canfield, D.E., Poulton, S.W., Narbonne, G.M., 2007. Late-Neoproterozoic Deep-Ocean Oxygenation and the Rise of Animal Life. Science, 315(5808): 92-95. doi: 10.1126/science.1135013 Chester, R., Hughes, M., 1967. A Chemical Technique for the Separation of Ferro-Manganese Minerals, Carbonate Minerals and Adsorbed Trace Elements from Pelagic Sediments. Chemical Geology, 2: 249-262. doi: 10.1016/0009-2541(67)90025-3 Coale, K.H., Fitzwater, S.E., Gordon, R.M., et al., 1996. Control of Community Growth and Export Production by Upwelled Iron in the Equatorial Pacific Ocean. Nature, 379: 621-624. doi: 10.1038/379621a0 Cornell, R.M., Schwertmann, U., 2003. The Iron Oxides. Structure, Properties, Reactions, Occurrences, and Uses, Wiley-Vch, Weinheim. Davidson, C.M., Ferreire, P.C.S., Ure, A.M., 1999. Some Sources of Variability in Application of the Three-Stage Sequential Extraction Procedure Recommended by BCR to Industrially-Comtaminated Soil. Fresenius Journal of Analytical Chemistry, 363(5-6): 446-451. doi: 10.1007/s002160051220 Geider, R.J., 1999. Complex Lessons of Iron Uptake. Nature, 400: 815-816. doi: 10.1038/23582 Gibbs, R.J., 1973. Mechanisms of Trace Metal Transport in Rivers. Science, 180(4081): 71-73. doi: 10.1126/science.180.4081.71 Gibbs, R.J., 1977. Transport Phases of Transition Metals in the Amazon and Yukon Rivers. Bulletin of the Geological Society of America, 88(6): 829-843. doi: 10.1130/0016-7606 Jickells, T., An, Z., Andersen, K.K., et al., 2005. Global Iron Connections between Desert Dust, Ocean Biogeochemistry, and Climate. Science, 308(5718): 67-71. doi: 10.1126/science.1105959 Lopez-Sanchez, J., Rubio, R., Rauret, G., 1993. Comparison of Two Sequential Extraction Procedures for Trace Metal Partitioning in Sediments. International Journal of Environmental Analytical Chemistry, 51(1-4): 113-121. doi: 10.1080/03067319308027616 Lyons, T., Severmann, S., 2006. A Critical Look at Iron Paleoredox Proxies: New Insights from Modern Euxinic Marine Basins. Geochimica et Cosmochimica Acta, 70(23): 5698-5722. doi: 10.1016/j.gca.2006.08.021 Maldonado, M.T., Price, N.M., 2001. Reduction and Transport of Organically Bound Iron by Thalassiosira Oceanica (Bacillariophyceae). Journal of Phycology, 37(2): 298-310. doi: 10.1046/j.1529-8817.2001.037002298.x Mao, C.P., 2009. Geochemical and Mineralogical Studies of the Sediment (Suspended Sediment) in Changjiang River Drainage Basin. Nanjing University, Nanjing: 35-38 (in Chinese with English abstract). Martin, J.H., 1992, Iron a Limiting Factor in Oceanic Primary Productivity. In: Falkowski, P.G., Woodhead, A.D., eds., Primary Productivity and Biogeochemical Cycles in the Sea. Plenum Press, New York. Martin, J.M., Nirel, P., Thomas, A., 1987. Sequential Extraction Techniques: Promises and Problems. Marine Chemistry, 22(2-4): 313-341. doi: 10.1016/0304-4203(87)90017-X Nodwell, L.M., Price, N.M., 2001. Direct Use of Inorganic Colloidal Iron by Marine Mixotrophic Phytoplankton(Dissertation). Limnology and Oceanography, Quebec, Canada, 765-777. Passos, E.A., Alves, J.C., dos Santos, I.S., et al., 2010. Assessment of Trace Metals Contamination in Estuarine Sediments Using a Sequential Extraction Technique and Principal Component Analysis. Microchemical Journal, 96(1): 50-57. doi: 10.1016/j.microc.2010.01.018 Poulton, S.W., Canfield, D.E., 2005. Development of a Sequential Extraction Procedure for Iron: Implications for Iron Partitioning in Continentally Derived Particulates. Chemical Geology, 214(3-4): 209-221. doi: 10.1016/j.chemgeo.2004.09.003 Poulton, S.W., Fralick, P.W., Canfield, D.E., 2010. Spatial Variability in Oceanic Redox Structure 1.8 Billion Years Ago. Nature Geoscience, 3: 486-490. doi: 10.1038/ngeo889 Poulton, S.W., Raiswell, R., 2000. Solid Phase Associations, Oceanic Fluxes and the Anthropogenic Perturbation of Transition Metals in World River Particulates. Marine Chemistry, 72(1): 17-31. doi: 10.1016/S0304-4203(00)00060-8 Poulton, S.W., Raiswell, R., 2002. The Low-Temperature Geochemical Cycle of Iron: From Continental Fluxes to Marine Sediment Deposition. American Journal of Science, 302(9): 774-805. doi: 10.2475/ajs.302.9.774 Raiswell, R., 2006. Towards a Global Highly Reactive Iron Cycle. Journal of Geochemical Exploration, 88(1-3): 436-439. doi: 10.1016/j.gexplo.2005.08.098 Raiswell, R., 2011. Iron Transport from the Continents to the Open Ocean: The Aging-Rejuvenation Cycle. Elements, 7(2): 101-106. doi: 10.2113/gselements.7.2.101 Raiswell, R., Benning, L., Davidson, L., et al., 2008. Nanoparticulate Bioavailable Iron Minerals in Icebergs and Glaciers. Mineralogical Magazine, 72(1): 345-348. doi: 10.1180/minmag.2008.072.1.345 Raiswell, R., Buckley, F., Berner, R.A., et al., 1988. Degree of Pyritization of Iron as a Paleoenvironmental Indicator of Bottom-Water Oxygenation. Journal of Sedimentary Research, 58(5): 812-819. doi: 10.2475/ajs.298.3.219 Raiswell, R., Canfield, D.E., 1998. Sources of Iron for Pyrite Formation in Marine Sediments. American Journal of Science, 298(3): 219-245. doi: 10.2475/ajs.298.3.219 Raiswell, R., Canfield, D.E., 2012. The Iron Biogeochemical Cycle Past and Present. Geochem. Perspect, 1(1): 1-220. doi: 10.7185/geochempersp.1.1 Raiswell, R., Canfield, D.E., Berner, R.A., 1994. A Comparison of Iron Extraction Methods for the Determination of Degree of Pyritisation and the Recognition of Iron-Limited Pyrite Formation. Chemical Geology, 111(1-4): 101-110. doi: 10.1016/0009-2541(94)90084-1 Rudnick, R.L., Gao, S., 2003. Composition of the Continental Crust. In: Heinrich, D.H., Karl, K.T., eds., Treatise on Geochemistry. Elsevier, Oxford. doi: 10.1016/B0-08-043751-6/03016-4 Song, Z.L., Liu, C.Q., Peng, B., et al., 2004. Sequential Extraction (SEE) Technology and Its Applications to Sediment and Soil Element Speciation Studies. Geology-Geochemistry, 32(2): 70-77(in Chinese with English abstract). http://d.wanfangdata.com.cn/Periodical/dzdqhx200402014 Sunda, W.G., 2001. Bioavailability and Bioaccumulation of Iron in the Sea. In: Turner, D.R., Hunter, K.A., eds., The Biogeochemistry of Iron in Seawater. Wiley, New York. Takata, H., Kuma, K., Iwade, S., et al., 2004. Spatial Variability of Iron in the Surface Water of the Northwestern North Pacific Ocean. Marine Chemistry, 86(3-4): 139-157. doi: 10.1016/j.marchem.2003.12.007 Tessier, A., Campbell, P.G.C., Bisson, M., 1979. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Analytical Chemistry, 51(7): 844-851. doi: 10.1021/ac50043a017 Thomas, R., Ure, A., Davidson, C., et al., 1994. Three-Stage Sequential Extraction Procedure for the Determination of Metals in River Sediments. Analytica Chimica Acta, 286(3): 423-429. doi: 10.1016/0003-2670(94)85088-7 Trefry, J.H., Presley, B.J., 1982. Manganese Fluxes from Mississippi Delta Sediments. Geochimica et Cosmochimica Acta, 46(10): 1715-1726. doi: 10.1016/0016-7037(82)90112-0 Ure, A., Quevauviller, P., Muntau, H., et al., 1993. Speciation of Heavy Metals in Soils and Sediments. An Account of the Improvement and Harmonization of Extraction Techniques Undertaken under the Auspices of the BCR of the Commission of the European Communities. International Journal of Environmental Analytical Chemistry, 51(1-4): 135-151. doi: 10.1080/03067319308027619 Wang, Y.P., Huang, Y., Wang, S.M., et al., 2005. Chemical Speciation of Elements in Sediments and Soils and Their Sequential Extraction Process. Regional Geology of China, 24(8): 728-734 (in Chinese with English abstract). http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZQYD200508010.htm Wijsman, J.W.M., Middelburg, J.J., Heip, C.H.R., 2001. Reactive Iron in Black Sea Sediments: Implications for Iron Cycling. Marine Geology, 172: 167-180. doi: 10.1016/S0025-3227(00)00122-5 茅昌平, 2009. 长江流域沉积物(悬浮物)的地球化学和矿物学研究(博士学位论文). 南京: 南京大学. 宋照亮, 刘丛强, 彭渤, 等, 2004. 逐级提取(SEE) 技术及其在沉积物和土壤元素形态研究中的应用. 地球与环境, 32(2): 70-77. https://www.cnki.com.cn/Article/CJFDTOTAL-DZDQ200402014.htm 王亚平, 黄毅, 王苏明, 等, 2005. 土壤和沉积物中元素的化学形态及其顺序提取法. 地质通报, 24(8): 728-734. doi: 10.3969/j.issn.1671-2552.2005.08.009 -
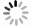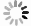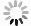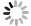








 下载:
下载: