Source Analysis of Sodium of Low-Salinity High-Sodium Geothermal Water in Huangshadong Geothermal Field from East Guangdong
-
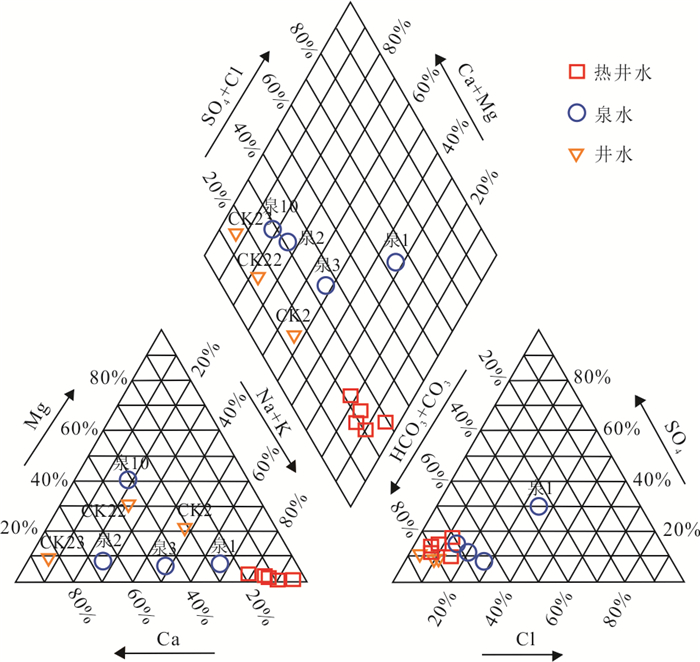
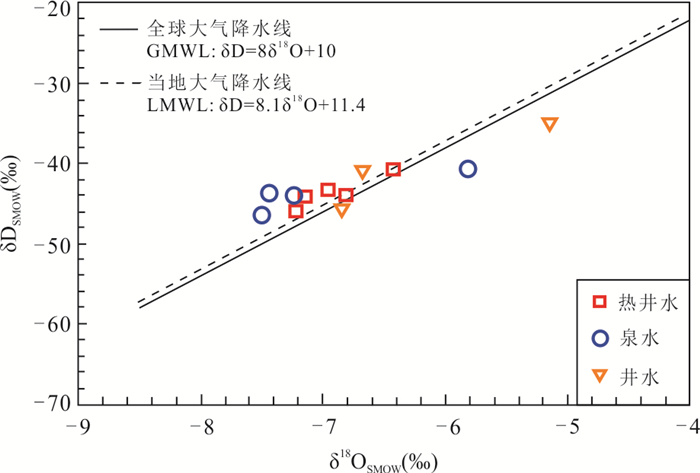
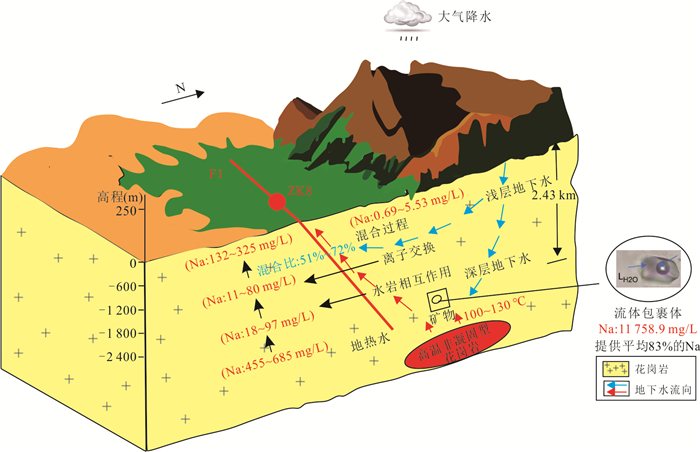
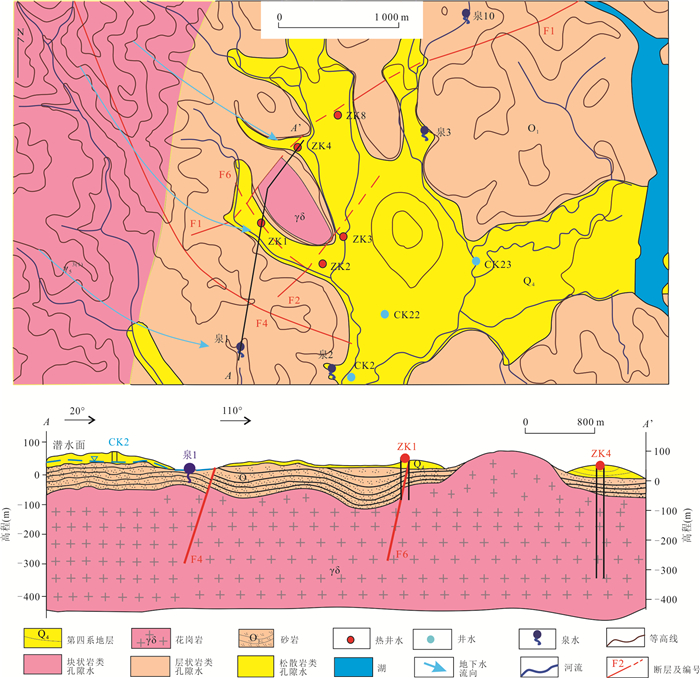
摘要: 高温地热系统中地热水中Na+含量一般超过300 mg/L,盐度也较大(TDS > 1000 mg/L).而在中低温地热系统中,低盐度地热水的Na+含量一般小于160 mg/L.但在广东黄沙洞中低温地热系统出露的地热水中Na+高达325.4 mg/L,TDS小于650 mg/L.经典的水文地球化学作用(矿物溶解、离子交换等)很难解释其成因.样品水化学结果表明,地热水水化学类型均为HCO3-Na型,钠含量高(平均值为240.06 mg/L).氢氧同位素结果表明地热水与浅层地下水均具有相同的大气来源,都是瑶坑山区大气降水补给.水化学地温计和多组分矿物平衡(MME)评估的热储温度为100~130 ℃,地热水循环深度最大为2.43 km.Cl-作为混合比计算指标揭示浅层地下水混入地热水的比例为51%~72%,深部地热水中Na+实际含量应该高达685.2 mg/L.水-岩相互作用模拟结果表明,矿物溶解和离子交换对地热水中Na+富集的贡献较小,也揭示出地热水中存在Na+的额外来源.花岗岩流体包裹体微小但广泛存在于结晶矿物颗粒之间,其中Na+含量平均值为11 758.9 mg/L.在地热水加热情况下,断裂和花岗岩裂隙网络层面及附近的流体包裹体膨胀破裂,流体混入到地热水中,为地热水提供了平均83%的Na+.因此,花岗岩流体包裹体可能是中低温地热系统低盐高钠地热水中Na+的主要来源.Abstract: The Na+ content in the geothermal water in the high-temperature geothermal system generally exceeds 300 mg/L, and the salinity is also large (TDS > 1 000 mg/L). In the medium- and low-temperature geothermal system, the Na+ content of low-salinity geothermal water is generally less than 160 mg/L. However, geothermal water with Na+ as high as 325.4 mg/L and TDS less than 650 mg/L was found in the Huangshadong medium-low temperature geothermal system in Guangdong. It is difficult to explain their formation with typical hydrogeochemical reactions (mineral dissolution, ion exchange, etc.). The water chemistry results show that the chemical types of geothermal water are all HCO3-Na type, with high sodium content (average 240.06 mg/L). The results of hydrogen and oxygen isotopes show that both geothermal water and shallow groundwater have the same atmospheric source which is in the Yaokeng mountainous area. According to the hydrochemical geothermometer and the multicomponent mineral equilibrium (MME), the thermal storage temperature is estimated to be 100-130 ℃ and the maximum circulation depth is 2.43 km. Cl- is used as a mixing ratio calculation indicator to reveal that the proportion of shallow groundwater mixed into geothermal water is 51%-72%, The actual content of Na+ in deep geothermal water should be as high as 685.2 mg/L. The simulation results of water-rock interaction indicate that mineral dissolution and ion exchange make a minor contribution to Na+ enrichment in geothermal water, and also reveal the existence of additional sources of Na+ in geothermal water. Granite fluid inclusions are tiny but widespread among crystalline mineral grains, and its average Na+ content is 11 758.9 mg/L. In the case of geothermal heating, the fluid inclusions at and near the fracture and granite fissure network expand and rupture, and the fluid is mixed into the geothermal water, providing average of 83% of Na+ for the geothermal water. Therefore, granite fluid inclusions may be the main source of Na+ for the low-salinity and high-sodium geothermal water in medium-low temperature geothermal system.
-
表 1 广东黄沙洞地热田样品水化学和同位素组成(水化学组分单位:mg/L)
Table 1. Hydrochemical and stable isotopic characteristics of the sampled waters from Huangshadong geothermal field in Guangdong (mg/L)
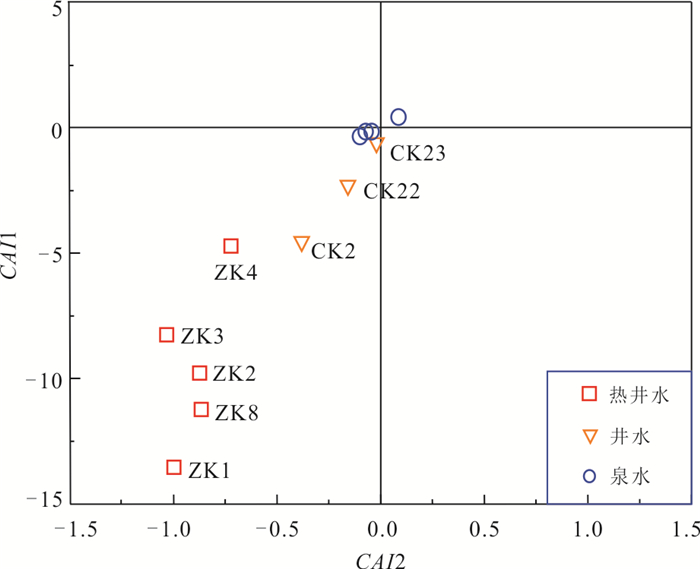
样品编号 类型 井深(m) 温度(℃) pH TDS HCO3‒ F‒ Cl‒ SO42‒ K+ Na+ Ca2+ Mg2+ SiO2 Li Al B Sr δD
(‰)δ18O
(‰)ZK8 热井水 591.5 89 7.54 638.6 631.4 15.585 57.240 112.893 26.090 325.40 12.41 0.403 142.000 1.215 0.066 0.600 0.256 ‒44.1 ‒6.8 ZK4 热井水 380 52 7.16 503.4 596.5 6.222 60.027 48.722 18.780 209.20 44.52 3.899 56.432 0.878 0.027 0.439 0.407 ‒40.8 ‒6.4 ZK1 热井水 100 58 7.80 301.1 285.6 15.244 14.429 36.006 7.207 131.80 12.41 0.260 95.173 0.255 0.041 0.073 0.301 ‒46.1 ‒7.2 ZK2 热井水 360 50 7.12 608.1 682.2 8.506 41.330 77.908 22.86 273.60 39.38 1.966 74.893 1.094 0.089 0.540 0.393 ‒44.2 ‒7.1 ZK3 热井水 310 47 7.69 614.6 666.3 9.084 34.621 69.798 21.950 260.30 35.86 1.919 65.481 1.060 0.015 0.535 0.420 ‒43.3 ‒6.9 泉10 泉水 22 5.64 17.2 25.4 0.066 2.942 3.983 0.302 0.69 1.61 0.926 10.538 ‒ 0.022 0.007 0.004 ‒43.9 ‒7.2 泉3 泉水 23 5.29 32.0 38.1 0.099 9.399 3.469 3.653 5.30 6.07 0.458 12.016 ‒ 0.107 0.007 0.017 ‒43.6 ‒7.4 泉2 泉水 22.5 6.11 54.3 50.8 0.218 8.757 5.953 2.230 5.53 15.00 1.042 8.617 ‒ 0.053 0.012 0.027 ‒40.5 ‒5.8 泉1 泉水 23 5.37 24.7 6.3 0.131 3.681 4.048 1.141 2.41 1.06 0.129 8.311 ‒ 0.086 0.006 0.003 ‒46.2 ‒7.5 CK2 井水 10.2 25 6.64 95.3 120.6 1.557 6.514 6.831 4.196 21.01 13.45 5.184 43.939 0.062 0.063 0.024 0.079 ‒35.2 ‒5.1 CK22 井水 9.6 26 6.58 134.6 88.8 0.616 4.512 5.162 2.042 8.39 16.19 6.400 36.195 ‒ 0.020 0.005 0.082 ‒41.3 ‒6.7 CK23 井水 11 26 7.73 128.4 158.6 0.910 5.052 13.527 1.909 3.81 51.29 3.209 21.018 ‒ 0.027 0.007 0.077 ‒46.0 ‒6.8 表 2 广东黄沙洞地热田地热水热储温度评估
Table 2. Calculated temperatures using geochemical geothermometers and multicomponent mineral equilibrium method
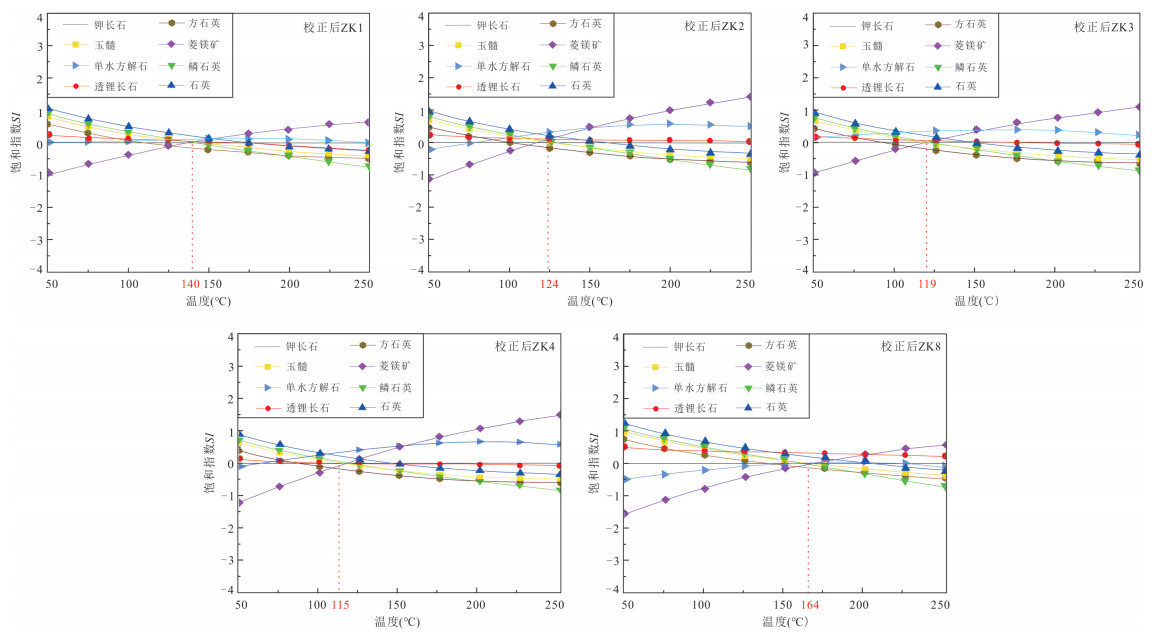
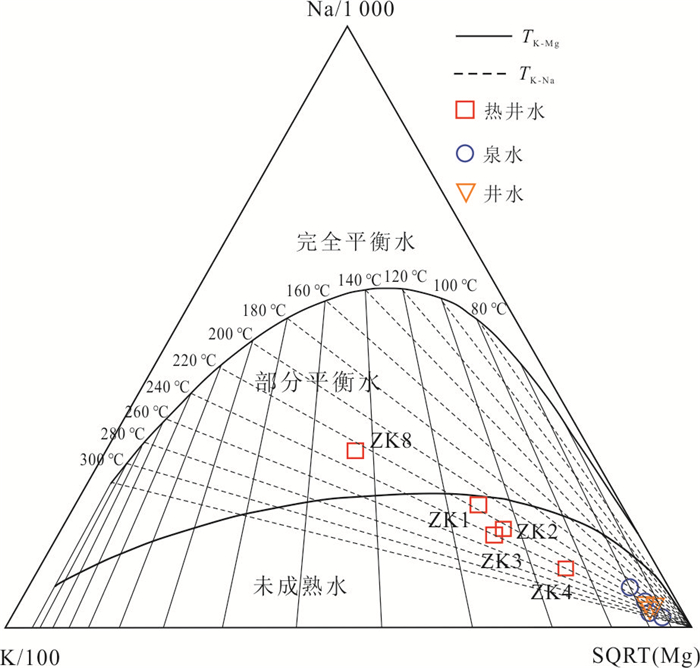
样品编号 测量温度
(℃)玉髓地温计 校正SiO2 Na-K- Ca-Mg Na-
KK-
MgNa-
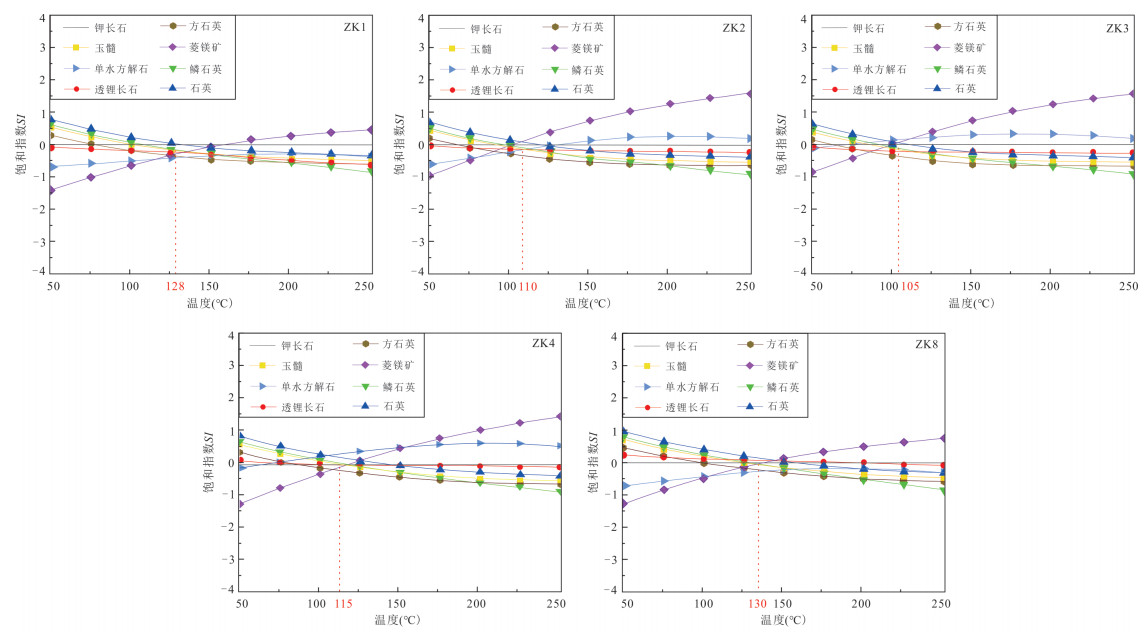
K-Ca多矿物组分平衡法 循环深度(km) ZK8 89 130.1 161.3 149.4 186.7 136.2 184.7 130 2.43 ZK4 52 78.9 108.6 132.3 198.1 93.0 171.4 100 1.75 ZK1 58 106.2 136.1 119.9 151.9 103.8 149.8 128 2.39 ZK2 50 93.2 122.9 136.2 190.8 107.8 173.4 110 2.27 ZK3 47 86.3 115.9 136.3 191.7 107.0 173.8 105 1.87 表 3 广东黄沙洞地热水中矿物溶解化学反应方程式
Table 3. Chemical reaction equation of mineral dissolution in thermal groundwater of Huangshadong geothermal field, Guangdong
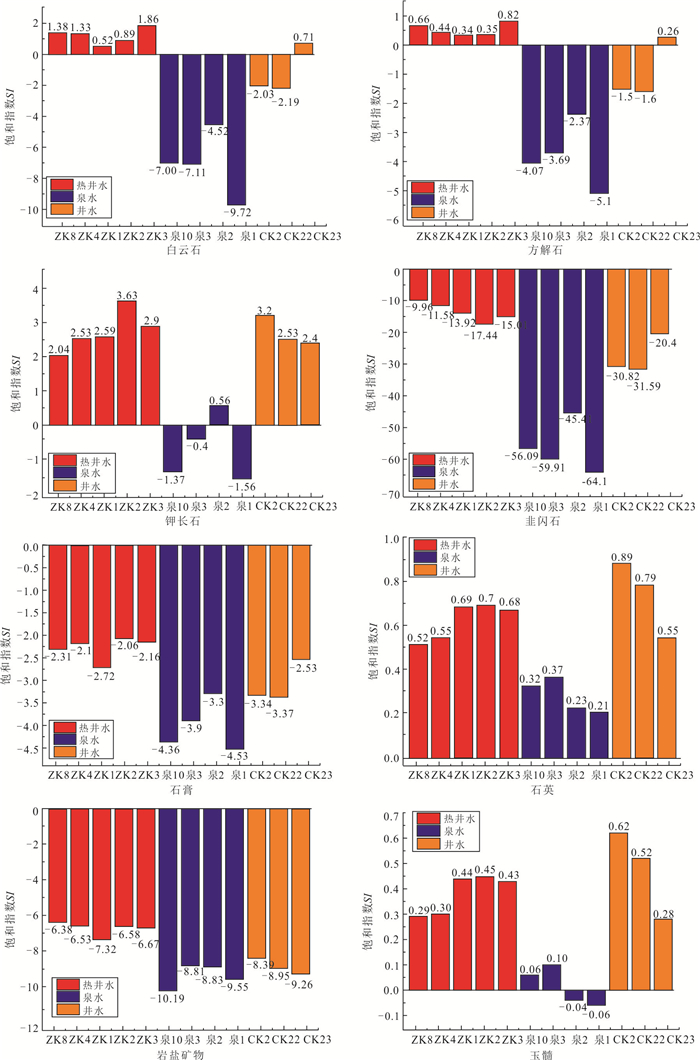
矿物名称 溶解反应方程式 角闪石 $ 2\mathrm{N}\mathrm{a}\mathrm{C}{\mathrm{a}}_{2}\mathrm{F}{\mathrm{e}}_{5}\left[\mathrm{S}{\mathrm{i}}_{7}\mathrm{A}\mathrm{l}{\mathrm{O}}_{22}\right]{\left(\mathrm{O}\mathrm{H}\right)}_{2}+3\mathrm{C}{\mathrm{O}}_{2}+39{\mathrm{H}}_{2}\mathrm{O}=\mathrm{A}{\mathrm{l}}_{2}\mathrm{S}\mathrm{i}{\mathrm{O}}_{5}{\left(\mathrm{O}\mathrm{H}\right)}_{4}+30\mathrm{H}\mathrm{C}{{\mathrm{O}}_{3}}^{-}+2\mathrm{N}{\mathrm{a}}^{+}+4\mathrm{C}{\mathrm{a}}^{2+}+10\mathrm{F}{\mathrm{e}}^{2+}+12{\mathrm{H}}_{4}\mathrm{S}\mathrm{i}{\mathrm{O}}_{4} $ 钾长石 $ 2\mathrm{K}\mathrm{A}\mathrm{l}\mathrm{S}{\mathrm{i}}_{3}{\mathrm{O}}_{8}+2{\mathrm{H}}_{2}\mathrm{C}{\mathrm{O}}_{3}+9{\mathrm{H}}_{2}\mathrm{O}=2{\mathrm{K}}^{+}+5{\mathrm{H}}_{2}\mathrm{S}\mathrm{i}{\mathrm{O}}_{4}+2\mathrm{H}\mathrm{C}{{\mathrm{O}}_{3}}^{-}+\mathrm{A}{\mathrm{l}}_{2}\mathrm{S}\mathrm{i}\mathrm{O}{}_{5}({\mathrm{O}\mathrm{H})}_{4}\left(\mathrm{高}\mathrm{岭}\mathrm{石}\right) $ 岩盐矿物 $ \mathrm{N}\mathrm{a}\mathrm{C}\mathrm{l}=\mathrm{N}{\mathrm{a}}^{+}+\mathrm{C}{\mathrm{l}}^{-} $ 方解石 $ \mathrm{C}\mathrm{a}\mathrm{C}{\mathrm{O}}_{3}+{\mathrm{H}}_{2}\mathrm{O}=\mathrm{C}{\mathrm{a}}^{2+}+\mathrm{H}\mathrm{C}{{\mathrm{O}}_{3}}^{-}+\mathrm{O}{\mathrm{H}}^{-} $ 白云石 $ \mathrm{C}\mathrm{a}\mathrm{M}\mathrm{g}(\mathrm{C}{\mathrm{O}}_{3}{)}_{2}+2{\mathrm{H}}_{2}\mathrm{O}=\mathrm{C}{\mathrm{a}}^{2+}+\mathrm{M}{\mathrm{g}}^{2+}+2\mathrm{H}\mathrm{C}{{\mathrm{O}}_{3}}^{-}+2\mathrm{O}{\mathrm{H}}^{-} $ 石膏 $ \mathrm{C}\mathrm{a}\mathrm{S}{\mathrm{O}}_{4}\cdot 2{\mathrm{H}}_{2}\mathrm{O}=\mathrm{C}{\mathrm{a}}^{2+}+\mathrm{S}{{\mathrm{O}}_{4}}^{2-}+2{\mathrm{H}}_{2}\mathrm{O} $ 表 4 黄沙洞地热田中模拟的地热水组分(mg/L)
Table 4. The water chemical composition (mg/L) of simulated geothermal water from Huangshadong geothermal field
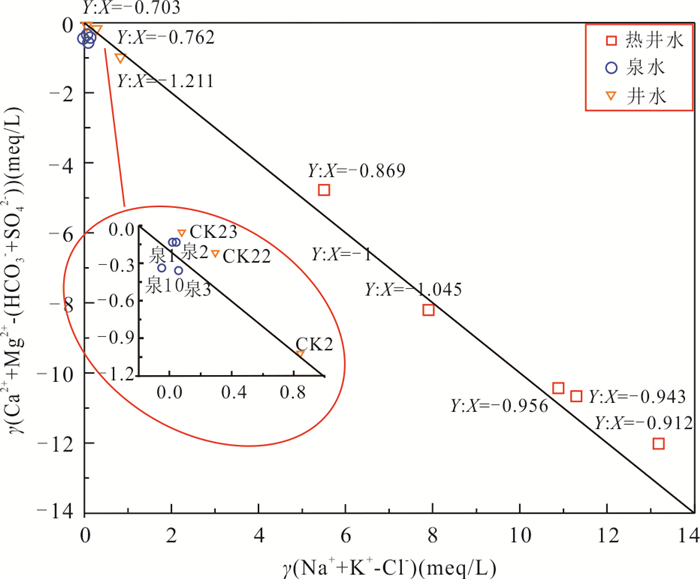
样品 HCO3‒ F‒ Cl‒ SO42‒ K+ Na+ Ca2+ Mg2+ SiO2 ZK8 379.91 5.45 111.01 74.73 5.03 71.16 45.16 0.83 135.42 ZK4 571.45 6.32 151.76 72.69 4.01 96.81 89.72 1.81 61.68 ZK1 125.17 5.95 28.90 65.46 4.70 17.91 63.32 1.14 96.18 ZK2 152.74 5.97 80.30 94.41 4.29 51.01 83.52 1.60 78.60 ZK3 175.25 6.19 66.14 70.55 4.15 41.91 80.32 1.57 70.02 表 5 华南黄沙洞地热田中不同地下水的钠含量(mg/L)及混合比
Table 5. The sodium content (mg/L) in different groundwaters and mixing ratio in geothemal water from Huangshadong geothermal field, South China
样品 混合比(Cl‒)
(%)混合比(Na+)
(%)矿物溶解平衡后地热水的Na+ 真实深部地热水的Na+ 矿物溶解平衡后地热水的Cl‒ 真实深部地热水的Cl‒ ZK8 53 ‒361 71.2 685.2 111.0 111.0 ZK4 64 ‒123 96.8 578.7 151.8 152.9 ZK1 72 ‒920 17.9 454.6 28.9 41.8 ZK2 51 ‒458 51.0 551.3 80.3 77.4 ZK3 53 ‒1045 41.9 546.3 66.1 63.8 表 6 地热水离子(mg/L)交换作用结果
Table 6. The ion (mg/L) exchange results of geothermal water
样品编号 混合后的地热水的Ca2+(热井水) 矿物溶解平衡后地热水的Ca2+ 混合前的地热水的Ca2+ 离子交换作用的Ca2+含量 离子交换作用的Na+含量 ZK8 12.41 45.16 18.15 27.01 54.02 ZK4 44.52 89.72 84.00 5.72 11.44 ZK1 12.41 63.32 23.42 39.90 79.81 ZK2 39.38 83.52 74.30 9.22 18.44 ZK3 35.86 80.32 67.66 12.66 25.32 表 7 石英矿物中单个流体包裹体的化学成分(10‒6)
Table 7. Chemical compositions (10‒6) of individual fluid inclusion trapped by quartz
Spot Li7 Na23 Mg24 Al27 K39 Sc45 Ti49 Mn55 Fe57 Cu63 Zn66 1 3 993 14 949 < LOD 12 517 < LOD 450.3 < LOD < LOD < LOD 1 192 < LOD 2 376 14 949 < LOD < LOD < LOD 48 < LOD < LOD < LOD 138 < LOD 3 3 330 14 949 < LOD 3 635 < LOD < LOD < LOD < LOD < LOD 1 721.5 < LOD 4 < LOD 11 022 1 243 839 2 678 < LOD < LOD 25 438 122 424 < LOD 1 891 5 9 194 14 949 < LOD < LOD < LOD 2 278.4 < LOD < LOD < LOD 3 344 < LOD 6 16 045 14 949 < LOD < LOD < LOD 2 640.4 < LOD < LOD < LOD 7 541 < LOD 7 2 836 1 562 < LOD < LOD 22 768 540 < LOD < LOD < LOD 1 425 < LOD 8 < LOD 13 958 52.1 < LOD 1 518 3.3 79.2 47 < LOD 6.9 6.7 9 < LOD 6 624 4 400 6 775 < LOD < LOD 4 416 62 549 271 070 < LOD 4 113.3 10 2 001 1 260 < LOD < LOD 23 282 293.2 < LOD < LOD < LOD 933 < LOD 11 < LOD 13 482 < LOD 9 239 2 489 < LOD 67.9 < LOD < LOD < LOD < LOD 12 < LOD 12 184 1 461 2 616 < LOD < LOD 5 180 13 791 114 088 < LOD 2 207.5 13 < LOD 10 423 2 392 2 757 < LOD < LOD 5 025 25 452 167 508 < LOD 2 446.9 14 < LOD 14 519 227 545 < LOD < LOD 218 1 889 12 639 < LOD 267.3 15 < LOD 11 540 1 802 4 107 < LOD < LOD 1 036 15 814 111 855 < LOD 1 745 16 < LOD 13 938 534 3 001 < LOD < LOD 752 6 487 55 285 < LOD 536.6 17 < LOD 14 644 161 262 < LOD < LOD 145.5 1 113 10 948 < LOD 224.1 注:据 Yang et al., 2019 ; < LOD表示低于检测限. -
Aydin, H., Karakuş, H., Mutlu, H., 2020. Hydrogeochemistry of Geothermal Waters in Eastern Turkey: Geochemical and Isotopic Constraints on Water-Rock Interaction. Journal of Volcanology and Geothermal Research, 390: 106708. https://doi.org/10.1016/j.jvolgeores.2019.106708 Bau, M., 1996. Controls on the Fractionation of Isovalent Trace Elements in Magmatic and Aqueous Systems: Evidence from Y/Ho, Zr/Hf, and Lanthanide Tetrad Effect. Contributions to Mineralogy and Petrology, 123(3): 323-333. https://doi.org/10.1007/s004100050159 Benmarce, K., Hadji, R., Zahri, F., et al., 2021. Hydrochemical and Geothermometry Characterization for a Geothermal System in Semiarid Dry Climate: The Case Study of Hamma Spring (Northeast Algeria). Journal of African Earth Sciences, 182: 104285. https://doi.org/10.1016/j.jafrearsci.2021.104285 Craig, H., 1953. The Geochemistry of the Stable Carbon Isotopes. Geochimica et Cosmochimica Acta, 3(2-3): 53-92. https://doi.org/10.1016/0016-7037(53)90001-5 Craig, H., 1961. Isotopic Variations in Meteoric Waters. Science, 133(3465): 1702-1703. https://doi.org/10.1126/science.133.3465.1702 Das, P., Maya, K., Padmalal, D., 2021. Hydrochemistry, Geothermometry and Origin of the Low Temperature Thermal Springs of South Konkan Region, India. Geothermics, 90: 101997. https://doi.org/10.1016/j.geothermics.2020.101997 Fournier, R. O., 1977. Chemical Geothermometers and Mixing Models for Geothermal Systems. Geothermics, 5(1-4): 41-50. https://doi.org/10.1016/0375-6505(77)90007-4 Giggenbach, W. F., 1988. Geothermal Solute Equilibria. Derivation of Na-K-Mg-Ca Geoindicators. Geochimica et Cosmochimica Acta, 52(12): 2749-2765. https://doi.org/10.1016/0016-7037(88)90143-3 Guo, W., Lin, X., Hu, S. H., 2020. Advances in LA-ICP-MS Analysis for Individual Fluid Inclusions and Applications. Earth Science, 45(4): 1362-1374 (in Chinese with English abstract). Guo, Y. Y., Lü, Z. C., Wang, G. C., et al., 2016. Hydrogeochemical Simulation of Groundwater in Eastern Fengfeng Mining Area. Coal Geology & Exploration, 44(6): 101-105 (in Chinese with English abstract). Han, D. M., Liang, X., Jin, M. G., et al., 2010. Evaluation of Groundwater Hydrochemical Characteristics and Mixing Behavior in the Daying and Qicun Geothermal Systems, Xinzhou Basin. Journal of Volcanology and Geothermal Research, 189(1-2): 92-104. https://doi.org/10.1016/j.jvolgeores.2009.10.011 Hu, S. B., He, L. J., Wang, J. Y., 2000. Heat Flow in the Continental Area of China: A New Data Set. Earth and Planetary Science Letters, 179(2): 407-419. https://doi.org/10.1016/S0012-821X(00)00126-6 Kuang, J., Qi, S. H., Wang, S., et al., 2020. Granite Intrusion in Huizhou, Guangdong Province and Its Geothermal Implications. Earth Science, 45(4): 1466-1480 (in Chinese with English abstract). Li, J., Liang, X., Chen, N. J., et al., 2017. Determination of Chemical Compositions of Pore Water in Clay-Rich Formations Using Geochemical Modeling. Hydrogeology & Engineering Geology, 44(1): 1-8 (in Chinese with English abstract). Li, N., 2020. Genetic Model of Karst Hot Water in Xiangxi River Basin and Inversion of Hydrogeological Parameters: A Case Study of Nanyang Hot Spring in Xingshan County, Hubei Province (Dissertation). China University of Geosciences, Wuhan (in Chinese with English abstract). Li, Y. G., Lin, W. J., Xing, L. X., et al., 2021. Estimation of Deep Geothermal Reservoir Temperature in Qabqa Area, Qinghai Province. Geology and Resources, 30(4): 479-484, 511 (in Chinese with English abstract). Liao, X., Jiang, H., Xu, Z. X., et al., 2020. Hydrogeochemical Characteristics and Genesis Mechanism of Geothermal Water in Awang, Eastern Tibet. Journal of Engineering Geology, 28(4): 916-924 (in Chinese with English abstract). Lin, W. R., Suzuki. S., Takahashi, X., et al., 2003. Fluid Inclusions and Thermal Microcracking in Inada Granite. Chinese Journal of Rock Mechanics and Engineering, 22(6): 899-904 (in Chinese with English abstract). Lu, H. Z., 1996. Magmatic, Fluid-Magmatic and Fluid Inclusions Studies on Granites, South China. Journal of Guilin Institute of Technology, 16(1): 1-13 (in Chinese with English abstract). Luo, L., Zhu, X., He, C. Y., et al., 2019. Study on the Genesis of Geothermal Fluid in Xianyang Geothermal Field. Geological Review, 65(6): 1422-1430 (in Chinese with English abstract). Mao, X. M., Wang, Y. X., Zhan, H. B., et al., 2015. Geochemical and Isotopic Characteristics of Geothermal Springs Hosted by Deep-Seated Faults in Dongguan Basin, Southern China. Journal of Geochemical Exploration, 158: 112-121. https://doi.org/10.1016/j.gexplo.2015.07.008 Mao, X. M., Zhu, D. B., Ndikubwimana, I., et al., 2021. The Mechanism of High-Salinity Thermal Groundwater in Xinzhou Geothermal Field, South China: Insight from Water Chemistry and Stable Isotopes. Journal of Hydrology, 593: 125889. https://doi.org/10.1016/j.jhydrol.2020.125889 Marques, J. M., Matias, M. J., Basto, M. J., et al., 2010. Hydrothermal Alteration of Hercynian Granites, Its Significance to the Evolution of Geothermal Systems in Granitic Rocks. Geothermics, 39(2): 152-160. https://doi.org/10.1016/j.geothermics.2010.03.002 Ni, P., Fan, H. R., Pan, J. Y., et al., 2021. Progress and Prospect of Fluid Inclusion Research in the Past Decade in China (2011-2020). Bulletin of Mineralogy, Petrology and Geochemistry, 40(4): 802-818, 1001 (in Chinese with English abstract). Nordstrom, D. K., Lindblom, S., Donahoe, R. J., et al., 1989. Fluid Inclusions in the Stripa Granite and Their Possible Influence on the Groundwater Chemistry. Geochimica et Cosmochimica Acta, 53(8): 1741-1755. https://doi.org/10.1016/0016-7037(89)90295-0 Pang, Z. H., Reed, M., 1998. Theoretical Chemical Thermometry on Geothermal Waters: Problems and Methods. Geochimica et Cosmochimica Acta, 62(6): 1083-1091. https://doi.org/10.1016/S0016-7037(98)00037-4 Qiu, X. L., Wang, Y., Wang, Z. Z., et al., 2018. Determining the Origin, Circulation Path and Residence Time of Geothermal Groundwater Using Multiple Isotopic Techniques in the Heyuan Fault Zone of Southern China. Journal of Hydrology, 567: 339-350. https://doi.org/10.1016/j.jhydrol.2018.10.010 Rowe, G. L., Brantley, S. L., 1993. Estimation of the Dissolution Rates of Andesitic Glass, Plagioclase and Pyroxene in a Flank Aquifer of Poás Volcano, Costa Rica. Chemical Geology, 105(1-3): 71-87. https://doi.org/10.1016/0009-2541(93)90119-4 Shen, Z. L., Wang, Y. X., 2002. Review and Outlook of Water-Rock Interaction Studies. Earth Science, 27(2): 127-133 (in Chinese with English abstract). doi: 10.3321/j.issn:1000-2383.2002.02.001 Su, Y., Ma, Z. Y., Liu, F., et al., 2007. Deuterium Excess Parameter Features Study on Thermal Groundwater of Xi'an and Xianyang. Coal Geology & Exploration, 35(3): 39-41 (in Chinese with English abstract). Sun, Z. X., Gao, B., Shvartsev, S., et al., 2017. The Thermal Water Geochemistry in Jiangxi Province (SE-China). Procedia Earth and Planetary Science, 17: 940-943. https://doi.org/10.1016/j.proeps.2017.01.031 Sun, Z. X., Zhu, Y. G., Zhang, W., 2004. Brief Review on Advancement of Geochemical Kinetical Studies of Mineral-Water Interactions. Journal of East China University of Technology (Natural Science), 27(1): 14-18 (in Chinese with English abstract). Wang, X. D., Liu, J. Q., Wang, X. W., 2008. Preliminary Study on Composition of Individual Fluid Inclusion in Minerals from Certain of W-Sn-Be Deposits, Nanling. Geology and Mineral Resources of South China, 24(3): 40-45 (in Chinese with English abstract). Wang, Z. F., Hao, R. J., Yang, H. B., et al., 2015. Research Progress on Water-Rock Interaction. Journal of Water Resources and Water Engineering, 26(3): 210-216 (in Chinese with English abstract). Wu, K. J., Ma, C. M., 2010. Geochemical Characteristics of Geothermal Water in Zhengzhou City. Geotechnical Investigation & Surveying, 38(5): 45-49 (in Chinese with English abstract). Xi, Y. F., Wang, G. L., Liu, S., et al., 2018. The Formation of a Geothermal Anomaly and Extensional Structures in Guangdong, China: Evidence from Gravity Analyses. Geothermics, 72: 225-231. https://doi.org/10.1016/j.geothermics.2017.11.009 Xun, Z., 2021. Evolution Characteristics of Zijin-Boluo Fault in Shiba-Huangshadong Area of Huizhou City and Its Relationship with Deep Geothermal Energy. Western Resources, (4): 106-108 (in Chinese with English abstract). Yan, X. X., Gan, H. N., Yue, G. F., 2019. Hydrogeochemical Characteristics and Genesis of Typical Geothermal Fileds from Huangshadong to Conghua in Guangdong. Geological Review, 65(3): 743-754 (in Chinese with English abstract). Yang, J. H., Kang, L. F., Liu, L., et al., 2019. Tracing the Origin of Ore-Forming Fluids in the Piaotang Tungsten Deposit, South China: Constraints from In-Situ Analyses of Wolframite and Individual Fluid Inclusion. Ore Geology Reviews, 111: 102939. https://doi.org/10.1016/j.oregeorev.2019.102939 Yu, B. C., 2011. Analysis of Active Tectonics and the Evaluation of Regional Crustal Stability Based on GIS in Pearl River Delta (Dissertation). China University of Geosciences, Wuhan (in Chinese with English abstract). Zhang, M., Kuang, J., Xiao, Z. C., et al., 2021. Geological Evolution since the Yanshanian in Huizhou, Guangdong Province: New Implications for the Tectonics of South China. Earth Science, 46(1): 242-258 (in Chinese with English abstract). Zhang, M. Z., Guo, Q. H., Liu, M. L., et al., 2023. Geochemical Characteristics and Formation Mechanisms of the Geothermal Waters in the Xinzhou Basin, Shanxi Province. Earth Science, 48(3): 973-987 (in Chinese with English abstract). 郭伟, 林贤, 胡圣虹, 2020. 单个流体包裹体LA-ICP-MS分析及应用进展. 地球科学, 45(4): 1362-1374 doi: 10.3799/dqkx.2019.199 郭钰颖, 吕智超, 王广才, 等, 2016. 峰峰矿区东部地下水水文地球化学模拟. 煤田地质与勘探, 44(6): 101-105. 旷健, 祁士华, 王帅, 等, 2020. 广东惠州花岗岩体及其地热意义. 地球科学, 45(4): 1466-1480. doi: 10.3799/dqkx.2019.128 李静, 梁杏, 陈乃嘉, 等, 2017. 地球化学模拟方法确定黏性土孔隙水化学组分. 水文地质工程地质, 44(1): 1-8. 李娜, 2020. 香溪河流域岩溶热水成因模式及水文地质参数反演研究: 以湖北省兴山县南阳温泉为例(博士学位论文). 武汉: 中国地质大学. 李永革, 蔺文静, 邢林啸, 等, 2021. 青海省恰卜恰地区深部热储温度估算. 地质与资源, 30(4): 479-484, 511. 廖昕, 蒋翰, 徐正宣, 等, 2020. 西藏东部阿旺地下热水化学特征及其成因初探. 工程地质学报, 28(4): 916-924. 林为人, 铃木舜一, 高桥学, 等, 2003. 稻田花岗岩中的流体包裹体及由其导致高温条件下微小裂纹的形成. 岩石力学与工程学报, 22(6): 899-904. 卢焕章, 1996. 华南花岗岩的岩浆与岩浆-流体包裹体及其意义. 桂林工学院学报, 16(1)1-13 罗璐, 朱霞, 何春艳, 等, 2019. 陕西咸阳地热田地热流体成因研究. 地质论评, 65(6): 1422-1430. 倪培, 范宏瑞, 潘君屹, 等, 2021. 流体包裹体研究进展与展望(2011-2020). 矿物岩石地球化学通报, 40(4): 802-818, 1001. 沈照理, 王焰新, 2002. 水-岩相互作用研究的回顾与展望. 地球科学, 27(2): 127-133. http://www.earth-science.net/article/id/1078 苏艳, 马致远, 刘方, 等, 2007. 西安、咸阳地下热水氘过量参数研究. 煤田地质与勘探, 35(3): 39-41. 孙占学, 朱永刚, 张文, 2004. 矿物-水反应的地球化学动力学研究进展. 东华理工学院学报, 27(1): 14-18. 王晓地, 刘家齐, 汪雄武, 2008. 南岭某些钨锡铍矿床中单个流体包裹体成分初步研究. 华南地质与矿产, 24(3): 40-45. 王周锋, 郝瑞娟, 杨红斌, 等, 2015. 水岩相互作用的研究进展. 水资源与水工程学报, 26(3): 210-216. 吴孔军, 马传明, 2010. 郑州市地下热水地球化学特征. 工程勘察, 38(5): 45-49. 荀忠, 2021. 惠州市石坝-黄沙洞地区紫金-博罗断裂演化特征及其与深层地热的关系. 西部资源, (4): 106-108. 闫晓雪, 甘浩男, 岳高凡, 2019. 广东惠州-从化典型地热田水文地球化学特征及成因分析. 地质论评, 65(3): 743-754. 于彬春, 2011. 基于GIS的珠江三角洲地区活动构造分析及区域地壳稳定性评价(硕士学位论文). 武汉: 中国地质大学. 张敏, 旷健, 肖志才, 等, 2021. 广东惠州燕山期以来地质构造演化: 对华南构造的新启示. 地球科学, 46(1): 242-258. doi: 10.3799/dqkx.2020.016 张梦昭, 郭清海, 刘明亮, 等, 2023. 山西忻州盆地地热水地球化学特征及其成因机制. 地球科学, 48(3): 973-987. doi: 10.3799/dqkx.2022.087 -








 下载:
下载: